This is a SUPER easy guide on Xenon element.
In fact, the table mentioned below is the perfect information box (Which gives you every single detail about the Xenon element in Periodic table.)
So if you want to know anything about Xenon element, then this guide is for you.
Let’s finish this very quickly.
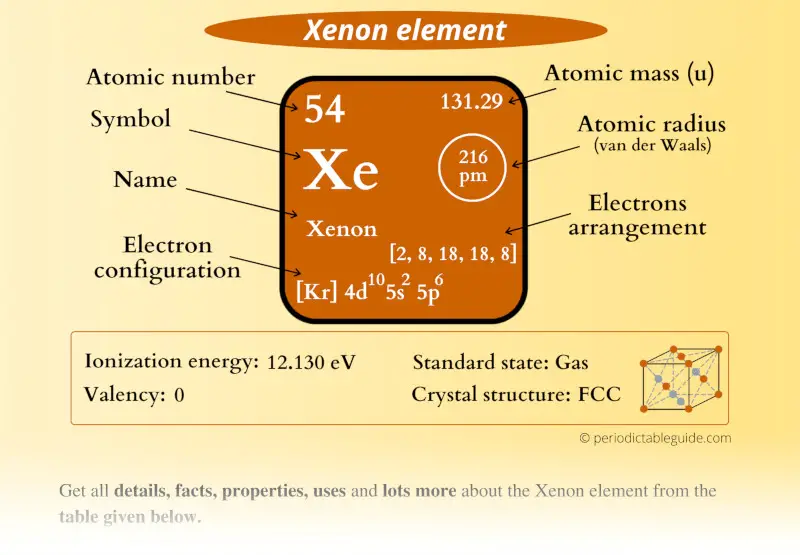
Xenon Element (Xe) Information
| Appearance | Colorless gas |
| State (at STP) | Gas |
| Position in Periodic table | 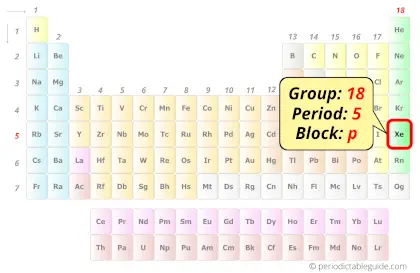 Group: 18, Period: 5, Block: p |
| Category |  Noble gases |
| Atomic number or Protons | 54 |
| Neutrons | 77 |
| Electrons | 54 |
| Symbol | Xe |
| Atomic mass |  131.29 u |
| Electrons arrangement or Bohr model | 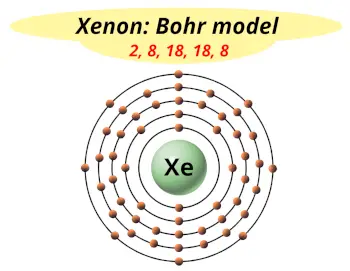 2, 8, 18, 18, 8 |
| Electronic configuration | [Kr] 4d10 5s2 5p6 |
| Atomic radius | 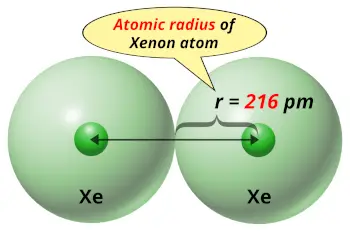 216 picometers (van der Waals radius) |
| Valence electrons | 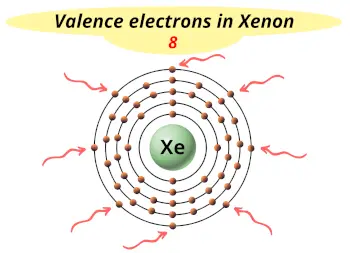 8 |
| 1st Ionization energy | 12.130 eV |
| Crystal structure | 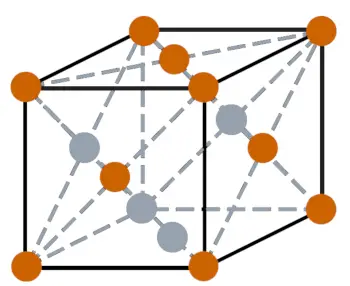 FCC (Face centered cubic) |
| Melting point | 161.4 K or -111.7 °C or -169.1 °F |
| Boiling point | 165 K or -108 °C or -162.5 °F |
| Density | 5.9 g/L |
| Main isotope | 129Xe (26.4%) and 132Xe (26.9%) |
| Who discovered Xenon and when? |  William Ramsey and Morris Travers (in 1898) |
| CAS number | 7440-63-3 |
Xenon in Periodic table
Xenon element is in group 18 and period 5 of the Periodic table. Xenon is the p-block element and it belongs to noble gases group.
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba | La* | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| *Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| **Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
←Move to: Iodine (I) element – Periodic Table
→Move to: Cesium (Cs) element – Periodic Table
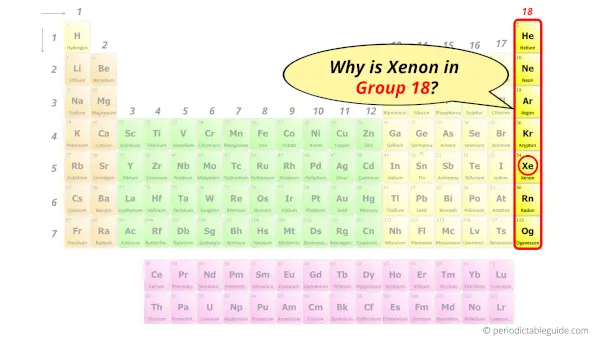
Why is Xenon in Group 18?
Do you know, how many electrons can be accommodated in the first shell, second shell, third shell, fourth shell, etc…?
Here is the table showing the capacity of orbits to hold electrons.
Number of electrons in shells.
| Orbit / Shell (n) | Maximum no. of electrons this orbit can hold (2 × n2) |
| K shell, n = 1 | 2 × 1² = 2 |
| L shell, n = 2 | 2 × 2² = 8 |
| M shell, n = 3 | 2 × 3² = 18 |
| N shell, n = 4 | 2 × 4² = 32 |
| . . . | . . . |
Thus,
- 1st shell can hold 2 electrons.
- 2nd shell can hold 8 electrons.
- 3rd shell can hold 18 electrons.
- 4th shell can hold 32 electrons, and so on.
Now the atomic number of Xenon (Xe) is 54.
Hence the electron arrangement in Xenon is 2, 8, 18, 18, 8. And the electron configuration of Xenon is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6.
This electron arrangement and electron configuration indicates that the outermost orbit (i.e orbit number 5) of Xenon atom has 8 electrons.
Hence, it lies in group 18.
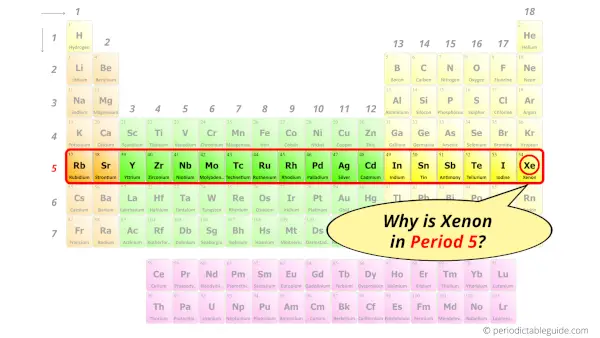
Why is Xenon in Period 5?
Let me ask you a question.
How many shells does xenon have?
It’s 5. Right?
You have already seen the bohr model of xenon atom in the above table.
From the Bohr model, it can be found that the number of orbits or shells in xenon is 5. Hence, as xenon has 5 orbits, it lies in period 5 of the Periodic table.
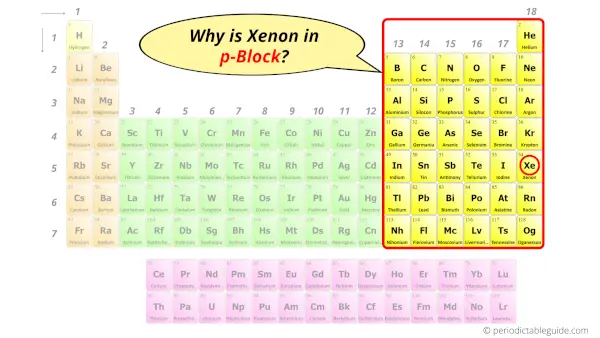
Why is Xenon in p-block?
Before knowing this reason, first of all I want to ask you a simple question.
How can you determine the blocks-wise position of elements?
The simple answer: The elements will lie in the s, p, d or f block will completely depend upon the subshell in which the last electron will enter.
For example; the electron configuration of Xenon is [Kr] 4d10 5s2 5p6.
So the last electron of Xenon enters the p-subshell or p-orbital.
Hence, Xenon is the p-block element.
7 Interesting facts about Xenon
Interesting facts about xenon element are mentioned below.
- The name xenon was derived from a Greek word “xenos” meaning stranger.
- The proportion of xenon present in earth’s atmosphere is very less and it’s around 1 part per 20 million only.
- Xenon is also present in the atmosphere of mars with only 0.08 ppm.
- Xenon is a very expensive noble gas as its availability is very less.
- Xenon was discovered by William Ramsey and Morris Travers (in 1898).
- Xenon gas is approximately 4 times heavier than air.
- The solidified xenon appears sky blue in color. (Note: It is very difficult to solidify xenon gas as it requires extremely high pressure).
Properties of Xenon
The physical and chemical properties of xenon element are mentioned below.
Physical properties of Xenon
Physical properties of xenon are mentioned below.
- Xenon is odorless, tasteless and colorless gas.
- When xenon is subjected to electrical discharge, it glows with blue or lavender light.
- The melting point of xenon is 161.4 K and its boiling point is 165 K.
- The atomic mass of xenon is 131.29 u and its density is 5.9 g/L.
- The crystal structure of xenon is FCC (Face centered cubic).
- Xenon has many isotopes, but out of them 129Xe and 132Xe exist in more proportions. The isotope 129Xe is approximately 26.4% and the isotope 132Xe is approximately 26.9%.
Chemical properties of Xenon
Chemical properties of xenon are mentioned below.
- Xenon has 8 valence electrons and is chemically inert, but it does form some compounds with very reactive elements like fluorine and other elements. (More than 100 compounds of xenon have been created till now).
- The electrons arrangement in xenon is 2, 8, 18, 18, 8 and its electron configuration is [Kr] 4d10 5s2 5p6. This indicates that it has 8 valence electrons (i.e electrons in its outermost orbit).
- The compounds of xenon act as an oxidizing agent and are highly toxic.
- The first ionization energy of xenon is 12.130 eV.
Uses of Xenon
Uses of xenon are mentioned below.
- As xenon glows with blue or lavender light on electrical discharge, it is used in stroboscopic lamps, flash lamps, etc.
- Xenon is also used in headlights of vehicles which gives a bluish glow.
- Xenon is also used in filling television and radio tubes.
- In medical applications, xenon gas is used as an anesthetic gas.
- Xenon finds its applications in laser manufacturing too.
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
External resources:
- Xenon – Element information, properties and uses | Periodic Table. (n.d.). Xenon – Element Information, Properties and Uses | Periodic Table. https://www.rsc.org/periodic-table/element/54/xenon
- Xenon – Wikipedia. (2010, May 6). Xenon – Wikipedia. https://en.wikipedia.org/wiki/Xenon
- P. (n.d.). Xenon | Xe (Element) – PubChem. Xenon | Xe (Element) – PubChem. https://pubchem.ncbi.nlm.nih.gov/element/Xenon
- It’s Elemental – The Element Xenon. (n.d.). It’s Elemental – the Element Xenon. https://education.jlab.org/itselemental/ele054.html
