This is a SUPER easy guide on Silicon element.
In fact, the table mentioned below is the perfect information box (Which gives you every single detail about the Silicon element in Periodic table.)
So if you want to know anything about Silicon element, then this guide is for you.
Let’s finish this very quickly.
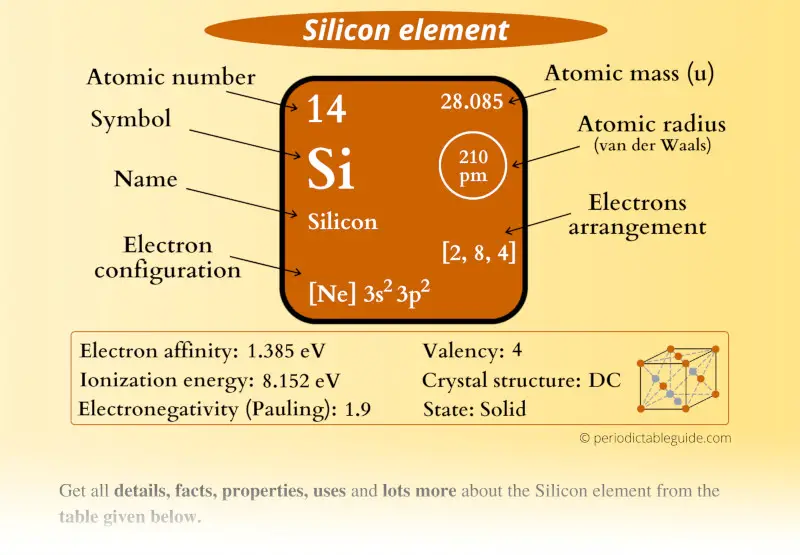
Silicon Element (Si) Information
| Appearance | Crystalline reflective solid with bluish-tinged faces |
| State (at STP) | Solid |
| Position in Periodic table | Group: 14, Period: 3, Block: p |
| Category | Metalloids (Carbon group) |
| Atomic number or Protons | 14 |
| Neutrons | 14 |
| Electrons | 14 |
| Symbol | Si |
| Atomic mass | 28.085 u |
| Electrons arrangement or Bohr model | 2, 8, 4 |
| Electronic configuration | [Ne] 3s2 3p2 |
| Atomic radius | 210 picometers (van der Waals radius) |
| Valence electrons | 4 |
| 1st Ionization energy | 8.152 eV |
| Electronegativity | 1.9 (Pauling scale) |
| Crystal structure | DC (Diamond cubic) |
| Melting point | 1687 K or 1414 °C or 2577 °F |
| Boiling point | 3538 K or 3265 °C or 5909 °F |
| Density | 2.33 g/cm3 |
| Main isotope | 28Si |
| Who discovered Silicon and when? | Jons Jacob Berzelius in 1823 |
| CAS number | 7440-21-3 |
Silicon in Periodic table
Silicon element is in group 14 and period 3 of the Periodic table. Silicon is the p-block element and it belongs to carbon group.
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba | La* | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| *Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| **Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
←Move to: Aluminum (Al) element – Periodic Table
→Move to: Phosphorus (P) element – Periodic Table
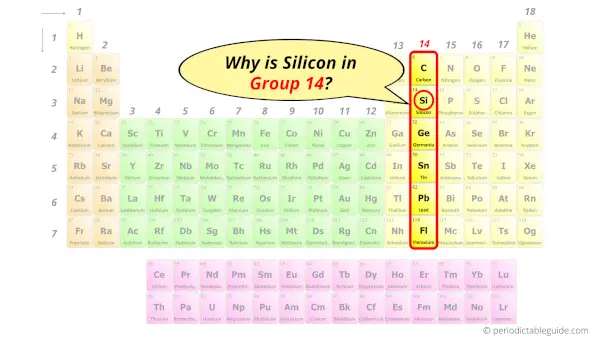
Why is Silicon in Group 14?
Do you know, how many electrons can be accommodated in the first shell, second shell, third shell, fourth shell, etc…?
Here is the table showing the capacity of orbits to hold electrons.
Number of electrons in shells.
| Orbit / Shell (n) | Maximum no. of electrons this orbit can hold |
| K shell, n = 1 | 2 × 1² = 2 |
| L shell, n = 2 | 2 × 2² = 8 |
| M shell, n = 3 | 2 × 3² = 18 |
| N shell, n = 4 | 2 × 4² = 32 |
Thus,
- 1st shell can hold 2 electrons.
- 2nd shell can hold 8 electrons.
- 3rd shell can hold 18 electrons.
- 4th shell can hold 32 electrons.
Now the atomic number of silicon (Si) is 14.
Hence the silicon element has electrons arrangement 2, 8, 4.
This electron arrangement indicates that the outermost orbit of Silicon element (Si) has 4 electrons.
Hence, it lies in group 14.
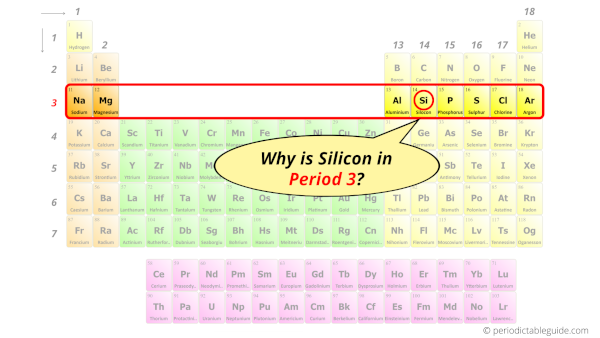
Why is Silicon in Period 3?
Let me ask you a question.
How many shells does silicon have?
It’s 3. Right?
You have already seen the bohr model of silicon element in the above table.
From the Bohr model, it can be found that the number of orbits or shells in silicon is 3. Hence, as silicon has 3 orbits, it lies in period 3 of the Periodic table.
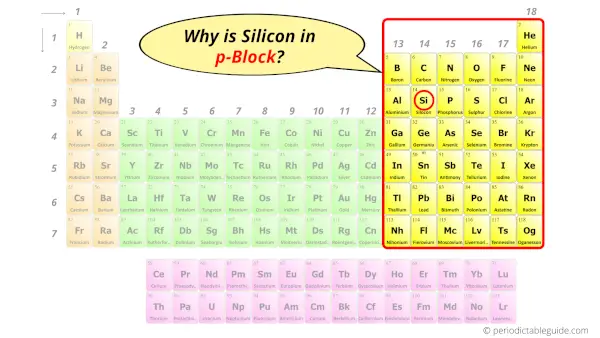
Why is Silicon in p-block?
Before knowing this reason, first of all I want to ask you a simple question.
How can you determine the blocks-wise position of elements?
The simple answer: The elements will lie in the s, p, d or f block will completely depend upon the subshell in which the last electron will enter.
For example; the electron configuration of silicon is [Ne] 3s2 3p2.
So the last electron of silicon enters the p-subshell or p-orbital.
Hence, silicon is the p-block element.
7 Interesting facts about Silicon
Interesting facts about silicon element are listed below.
- Silicon is the 2nd most abundant element present in the earth’s crust.
- Silicon conducts electricity like the metals. But this electrical conductivity increases with the increase in temperature. (The electrical conductivity of metals decreases with increasing temperature.)
- Silicon is not found naturally, but is always found as a compound with other elements.
- Silicon is also present in the meteorites.
- Silicon occupies almost 27% of the earth’s crust. This is because most of the compounds found in the earth’s crust contain silicon element in them.
- Silicon is important for plants as it strengthens the plants cell wall.
- Inhaling silicon dust causes a dangerous lung disease known as silicosis.
Properties of Silicon
The physical and chemical properties of silicon elements are listed below.
Physical properties of Silicon
Physical properties of silicon are listed below.
- Silicon is a crystalline reflective solid having bluish-tinged faces.
- Silicon has higher density in its liquid state as compared to that in solid state.
- The hardness of silicon carbide (SiC) is very high. This hardness is almost near to the hardness of diamond, but less than that of diamond (Mohs hardness of silicon carbide is between 9 to 9.5, while that of diamond is 10).
- Silicon has 3 isotopes, out of which 28Si is a stable one.
- Silicon has a melting point of 2577 °F and a boiling point of 5909 °F.
Chemical properties of Silicon
Chemical properties of silicon are listed below.
- Silicon shows the chemical properties of metals as well as nonmetals. Hence it is classified as a metalloid on the periodic table. (The majority of the chemical properties of silicon are similar to that of nonmetals.)
- Silicon can form compounds with metals as well as nonmetals.
- Silicon reacts with strong alkalis to form silicates and liberates hydrogen gas.
Uses of Silicon
Uses of silicon are listed below.
- Silicon is a semiconductor material and hence it is used in making computer chips, solar cells, etc, which can conduct electricity at higher temperatures.
- Transistors which are used in most of the electronic devices from radios to iphones, uses silicon.
- Silicon is the main component present in ceramics, glass and bricks.
- Silicon is also used in the production of steels.
- Abrasives as well as cutting tools are also made from silicon carbide.
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
External resources:
- Silicon – Element information, properties and uses | Periodic Table. (n.d.). Silicon – Element Information, Properties and Uses | Periodic Table. https://www.rsc.org/periodic-table/element/14/silicon
- Silicon – Wikipedia. (2019, August 22). Silicon – Wikipedia. https://en.wikipedia.org/wiki/Silicon
- It’s Elemental – The Element Silicon. (n.d.). It’s Elemental – the Element Silicon. https://education.jlab.org/itselemental/ele014.html
- P. (n.d.). Silicon | Si (Element) – PubChem. Silicon | Si (Element) – PubChem. https://pubchem.ncbi.nlm.nih.gov/element/Silicon
