This is a SUPER easy guide on Lead element.
In fact, the table mentioned below is the perfect information box (Which gives you every single detail about the Lead element in Periodic table.)
So if you want to know anything about Lead element, then this guide is for you.
Let’s dive right into it!
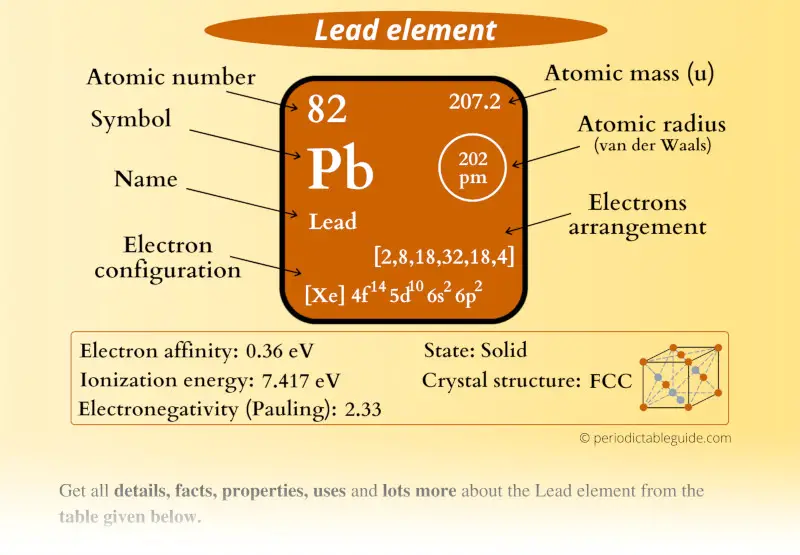
Lead Element (Pb) Information
| Appearance |  Metallic gray appearance |
| State (at STP) | Solid |
| Position in Periodic table | 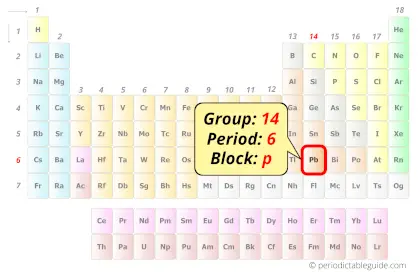 Group: 14, Period: 6, Block: p |
| Category | 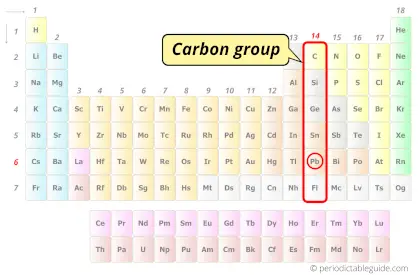 Carbon group |
| Atomic number or Protons | 82 |
| Neutrons | 125 |
| Electrons | 82 |
| Symbol | Pb |
| Atomic mass | 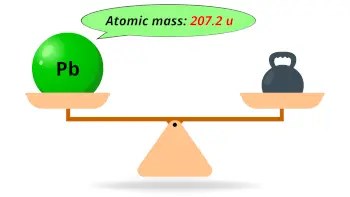 207.2 u |
| Electrons arrangement or Bohr model | 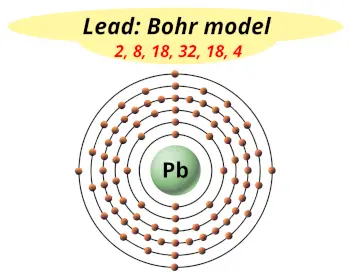 2, 8, 18, 32, 18, 4 |
| Electronic configuration | [Xe] 4f14 5d10 6s2 6p2 |
| Atomic radius | 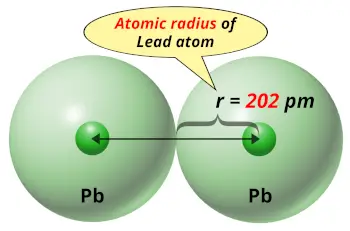 202 picometers (van der Waals radius) |
| Valence electrons | 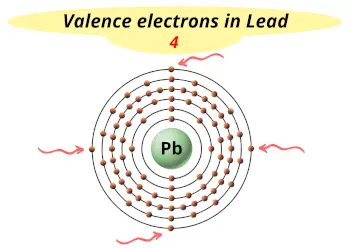 4 |
| 1st Ionization energy | 7.417 eV |
| Electronegativity | 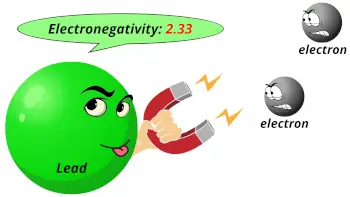 2.33 (Pauling scale) |
| Crystal structure | 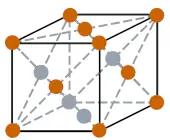 FCC (face centered cubic) |
| Melting point | 600.6 K or 327.4 °C or 621.4 °F |
| Boiling point | 2022 K or 1749 °C or 3180 °F |
| Density | 11.34 g/cm3 |
| Main isotope | 208Pb |
| CAS number | 7439-92-1 |
Lead in Periodic table
Lead element is in group 14 and period 6 of the Periodic table. Lead is the p-block element and it belongs to carbon group.
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba | La* | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| *Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| **Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
←Move to: Thallium (Tl) element – Periodic Table
→Move to: Bismuth (Bi) element – Periodic Table
Why is Lead in Group 14?
Do you know, how many electrons can be accommodated in the first shell, second shell, third shell, fourth shell, etc…?
Here is the table showing the capacity of orbits to hold electrons.
Number of electrons in shells.
| Orbit / Shell (n) | Maximum no. of electrons this orbit can hold (2 × n2) |
| K shell, n = 1 | 2 × 1² = 2 |
| L shell, n = 2 | 2 × 2² = 8 |
| M shell, n = 3 | 2 × 3² = 18 |
| N shell, n = 4 | 2 × 4² = 32 |
| . . . | . . . |
Thus,
- 1st shell can hold 2 electrons.
- 2nd shell can hold 8 electrons.
- 3rd shell can hold 18 electrons.
- 4th shell can hold 32 electrons.
Now the atomic number of Lead (Pb) is 82.
Hence the Lead element has electrons arrangement 2, 8, 18, 32, 18, 4.
This electron arrangement indicates that the outermost orbit of Lead element (Pb) has 4 electrons.
Hence, it lies in group 14.
Why is Lead in Period 6?
Let me ask you a question.
How many shells does lead have?
It’s 6. Right?
You have already seen the bohr model of lead atom in the above table.
From the Bohr model, it can be found that the number of orbits or shells in lead is 6. Hence, as lead has 6 orbits, it lies in period 6 of the Periodic table.
Why is Lead in p-block?
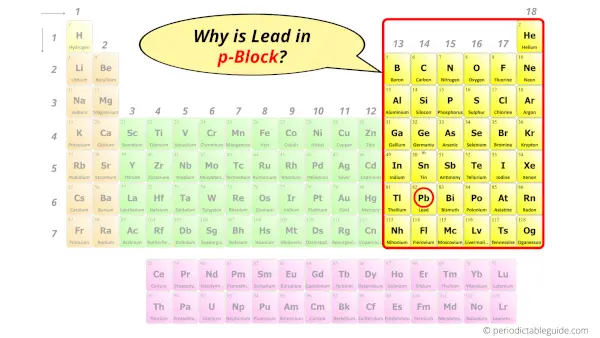
Before knowing this reason, first of all I want to ask you a simple question.
How can you determine the blocks-wise position of elements?
The simple answer: The elements will lie in the s, p, d or f block will completely depend upon the subshell in which the last electron will enter.
For example; the electron configuration of lead is [Xe] 4f14 5d10 6s2 6p2.
So the last electron of lead enters the p-subshell or p-orbital.
Hence, lead is the p-block element.
5 Interesting facts about Lead
Interesting facts about lead element are mentioned below.
- The concentration of lead in the earth’s crust is around 114 ppm by weight.
- Lead is primarily obtained from its ore lead sulfide (PbS). lead is also obtained from the ores of copper, silver and zinc.
- Lead is one of the elements that was discovered since ancient times.
- Lead is a soft metal and it can be cut with a knife also.
- Lead is a poisonous element. Too much lead in the human body can cause nervous problems as well as it also affects organs like kidney, heart, intestines, etc.
Properties of Lead
The physical and chemical properties of lead element are mentioned below.
Physical properties of Lead
Physical properties of lead are mentioned below.
- Lead is a solid metal at STP and it has a metallic grey appearance.
- Lead is a poor conductor of electricity.
- Lead is ductile as well as malleable metal. That means it can be drawn into thin wires and thin sheets.
- There are many isotopes of lead, but out of those isotopes the isotope 208Pb is the most abundant (having abundance of approximately 52.4%).
- Lead element has a lower melting point of 327.4 °C. And its boiling point is 1749 °C.
- The crystal structure of lead is FCC (i.e face centered cubic).
Chemical properties of Lead
Chemical properties of lead are mentioned below.
- When the lead is freshly cut, its surface is bluish grey. But if it is kept open in air, then it reacts with oxygen and starts tarnishing (which forms a grey oxide layer on it).
- The electron configuration of lead is [Xe] 4f14 5d10 6s2 6p2, which shows that the last electron enters the p-orbital. Because of this reason, it is classified as a p-block element on the periodic table.
- In the compound form, lead exists in its most common oxidation states +2 and +4. Apart from these oxidation states, other oxidation states also exist.
- The 1st ionization energy of lead is 7.417 eV.
- The electronegativity of lead is 2.33 on the Pauling scale.
Uses of Lead
Uses of lead are mentioned below.
- Most of the lead produced nowadays is used in manufacturing lead batteries.
- Lead is also used in solder materials as well as in covering materials for cables.
- In ancient times, lead was used in plumbing (as it is non corrosive). But due to its toxicity it is not used nowadays.
- Lead is also used in manufacturing radiation shields, bullets, etc.
- Lead acts as a superconductor at temperatures below 7.2 K.
Free Gift for you: Interactive Periodic Table
Let me tell you how this Interactive Periodic Table will help you in your studies.
1). You can effortlessly find every single detail about the elements from this single Interactive Periodic table.
2). You will get the detailed information about the periodic table which will convert a newbie into pro.
3). You will also get the HD images of the Periodic table (for FREE).
Checkout Interactive Periodic table and download it’s high resolution image now (It’s FREE)
External resources:
- Lead – Wikipedia. (2021, November 5). Lead – Wikipedia. https://en.wikipedia.org/wiki/Lead
- Lead – Element information, properties and uses | Periodic Table. (n.d.). Lead – Element Information, Properties and Uses | Periodic Table. https://www.rsc.org/periodic-table/element/82/lead
- P. (n.d.). Lead | Pb (Element) – PubChem. Lead | Pb (Element) – PubChem. https://pubchem.ncbi.nlm.nih.gov/element/Lead
- It’s Elemental – The Element Lead. (n.d.). It’s Elemental – the Element Lead. https://education.jlab.org/itselemental/ele082.html
- Learn about Lead | US EPA. (2013, February 12). US EPA. https://www.epa.gov/lead/learn-about-lead