This is a SUPER easy guide on Tantalum element.
In fact, the table mentioned below is the perfect information box (Which gives you every single detail about the Tantalum element in Periodic table.)
So if you want to know anything about Tantalum element, then this guide is for you.
Let’s finish this very quickly.
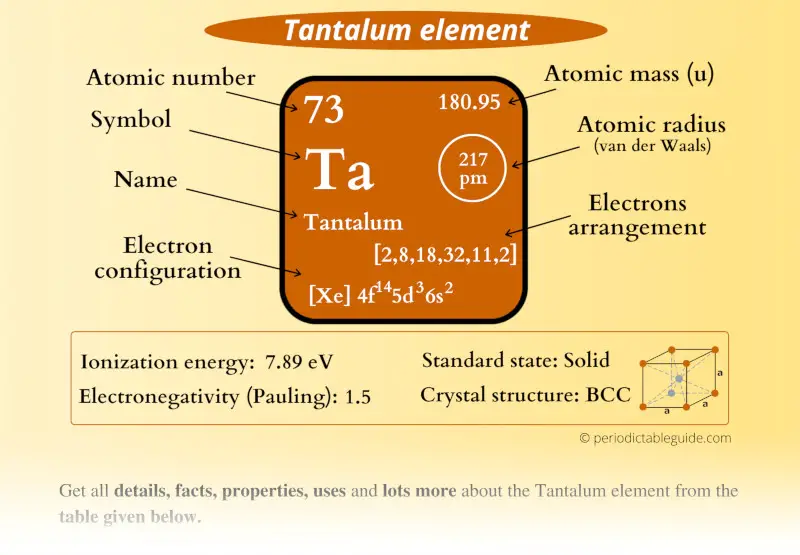
Tantalum Element (Ta) Information
| Appearance |  Gray metallic with bluish tint |
| State (at STP) | Solid |
| Position in Periodic table | 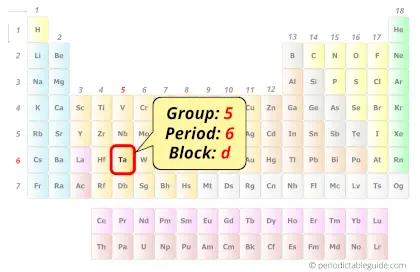 Group: 5, Period: 6, Block: d |
| Category | 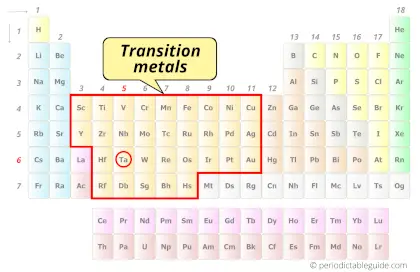 Transition metals |
| Atomic number or Protons | 73 |
| Neutrons | 108 |
| Electrons | 73 |
| Symbol | Ta |
| Atomic mass | 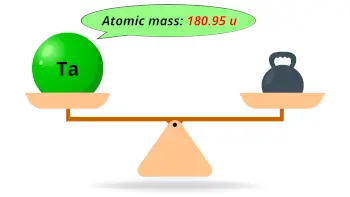 180.95 u |
| Electrons arrangement or Bohr model | 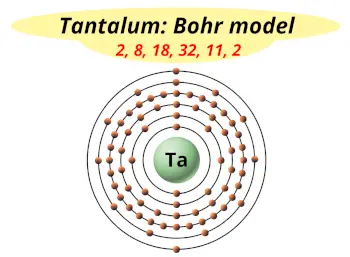 2, 8, 18, 32, 11, 2 |
| Electronic configuration | [Xe] 4f14 5d3 6s2 |
| Atomic radius | 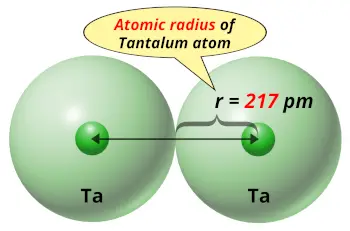 217 picometers (van der Waals radius) |
| 1st Ionization energy | 7.89 eV |
| Electronegativity | 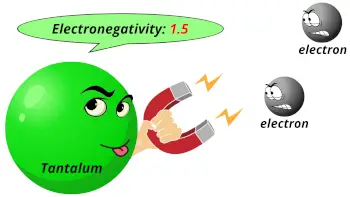 1.5 (Pauling scale) |
| Crystal structure | 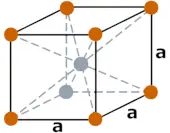 BCC (Body centered cubic) |
| Melting point | 3290 K or 3017 °C or 5463 °F |
| Boiling point | 5731 K or 5458 °C or 9856 °F |
| Density | 16.65 g/cm3 |
| Main isotope | 181Ta |
| Who discovered Tantalum and when? | Anders Gustaf Ekeberg (in 1802) |
| CAS number | 7440-25-7 |
Tantalum in Periodic table
Tantalum element is in group 5 and period 6 of the Periodic table. Tantalum is the d-block element and it belongs to transition metals group.
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba | La* | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| *Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| **Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
←Move to: Hafnium (Hf) element – Periodic Table
→Move to: Tungsten (W) element – Periodic Table
Why is Tantalum in Period 6?
Let me ask you a question.
How many shells does a tantalum atom have?
It’s 6. Right?
You have already seen the bohr model of tantalum atom in the above table.
From the Bohr model, it can be found that the number of orbits or shells in tantalum is 6. Hence, as tantalum has 6 orbits, it lies in period 6 of the Periodic table.
Why is Tantalum in d-block?
Before knowing this reason, first of all I want to ask you a simple question.
How can you determine the blocks-wise position of elements?
The simple answer: The elements will lie in the s, p, d or f block will completely depend upon the subshell in which the last electron will enter.
For example; the electron configuration of tantalum is [Xe] 4f14 5d3 6s2.
So the last electron of tantalum enters the d-subshell or d-orbital.
Hence, tantalum is the d-block element.
Is Tantalum a Transition Metal? Why?
Yes, Tantalum is a transition metal because it has incompletely filled d-orbital in its ground state.
Let me explain the exact meaning of this.
According to the definition of transition metals;
The element should compulsorily have incomplete d-orbitals, either in their ground state (M) or most common oxidation states (M1+, M2+, etc) then only they are called transition metals.
Now, the ground state of Tantalum means its normal state in which it has neither gained nor lost any electron/s.
So the ground state of Tantalum is Ta.
And the ground state electronic configuration of tantalum is [Xe] 4f14 5d3 6s2.
In this state, if we see the electron configuration of tantalum, then it possesses incomplete d-orbitals.
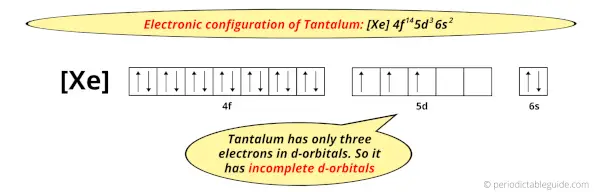
Because, there are only three electrons in the d-orbitals (here 5d orbitals).
In order to have the complete d-orbitals, there must be 10 electrons in it.
But in the ground state electronic configuration of tantalum, you can see that it has only 3 electrons in d-orbitals.
Thus, tantalum has incomplete d-orbitals.
And hence, as Tantalum has incomplete d-orbitals, it is considered as a transition metal.
5 Interesting facts about Tantalum
Interesting facts about tantalum element are mentioned below.
- The name “Tantalum” was derived from the name of the Greek mythological character “Tantalos”.
- Tantalum was discovered by Anders Gustaf Ekeberg in 1802.
- The concentration of tantalum in the earth’s crust is approximately 1.7 ppm by weight.
- Tantalum is mainly present in the minerals Columbite and Tantalite in the earth’s crust.
- Australia is the leading producer of tantalum in the world (According to a report of USGS).
Properties of Tantalum
The physical and chemical properties of tantalum element are mentioned below.
Physical properties of Tantalum
Physical properties of tantalum are mentioned below.
- Tantalum is a dense metal having a silvery gray metallic appearance with bluish tint.
- Tantalum is also a ductile metal that can be drawn into thin wires.
- Tantalum has a very high melting point and boiling point. The melting point of tantalum is 3017 °C and its boiling point is 5458 °C.
- Tantalum is also a good conductor of heat and electricity.
- Tantalum has many isotopes and out of those isotopes, the most abundant isotope is 181Ta (which has an abundance of approximately 99.9%).
- The crystal structure of tantalum is BCC (Body centered cubic).
Chemical properties of Tantalum
Chemical properties of tantalum are mentioned below.
- Tantalum is generally resistant to chemical reactions at room temperature.
- Tantalum is also resistive to corrosion because of the oxide layer formed on it.
- At room temperature, tantalum shows a chemical reaction with hydrofluoric acid.
Uses of Tantalum
Uses of tantalum are mentioned below.
- In electronic industries, tantalum is used in manufacturing resistors as well as capacitors.
- Tantalum is also used as an alloying element with other elements which increases the melting point of the alloy.
- Tantalum oxides which contain tantalum in it are used to make camera lenses. These camera lenses have a higher refractive index.
- As it is resistant to higher temperatures, it is used in parts of missiles as well as aircrafts.
Free Gift for you: Interactive Periodic Table
Let me tell you how this Interactive Periodic Table will help you in your studies.
1). You can effortlessly find every single detail about the elements from this single Interactive Periodic table.
2). You will get the detailed information about the periodic table which will convert a newbie into pro.
3). You will also get the HD images of the Periodic table (for FREE).
Checkout Interactive Periodic table and download it’s high resolution image now (It’s FREE)
External resources:
- Tantalum – Wikipedia. (2008, September 5). Tantalum – Wikipedia. https://en.wikipedia.org/wiki/Tantalum
- Tantalum – Element information, properties and uses | Periodic Table. (n.d.). Tantalum – Element Information, Properties and Uses | Periodic Table. https://www.rsc.org/periodic-table/element/73/tantalum
- It’s Elemental – The Element Tantalum. (n.d.). It’s Elemental – the Element Tantalum. https://education.jlab.org/itselemental/ele073.html
- P. (n.d.). Tantalum | Ta (Element) – PubChem. Tantalum | Ta (Element) – PubChem. https://pubchem.ncbi.nlm.nih.gov/element/Tantalum
- Virginia Energy – Geology and Mineral Resources – Tantalum. (n.d.). Virginia Energy – Geology and Mineral Resources – Tantalum. https://www.energy.virginia.gov/geology/tantalum.shtml