This is a SUPER easy guide on hydrogen element.
In fact, the table mentioned below is the perfect information box (Which gives you every single detail about the Hydrogen element in Periodic table.)
So if you want to know anything about hydrogen element, then this guide is for you.
Let’s finish this very quickly.
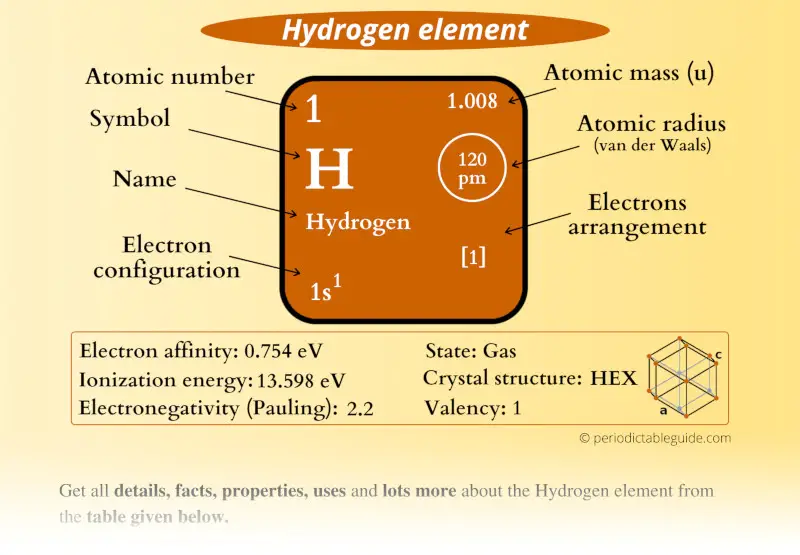
Hydrogen Element (H) Information
| Appearance | Colorless gas |
| State (at STP) | Gas |
| Position in Periodic table | 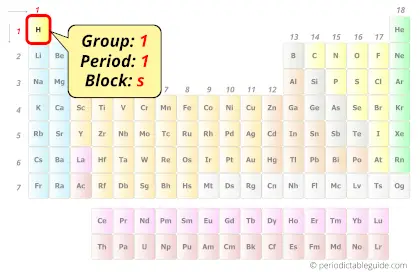 Group: 1, Period: 1, Block: s |
| Category | Other Nonmetals |
| Atomic number or Protons | 1 |
| Neutrons | 0 |
| Electrons | 1 |
| Symbol | H |
| Atomic mass | 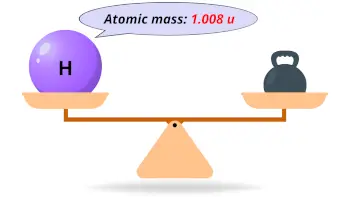 1.008 u |
| Electrons arrangement or Bohr model | 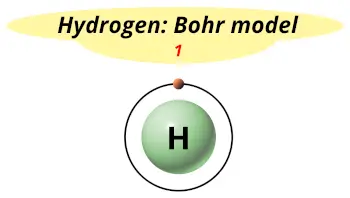 1 |
| Electronic configuration | 1s1 |
| Atomic radius | 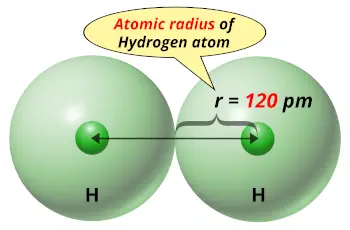 120 picometers (van der Waals radius) |
| Valence electrons | 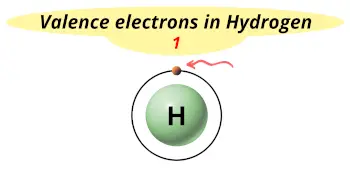 1 |
| 1st Ionization energy | 13.598 eV |
| Electronegativity | 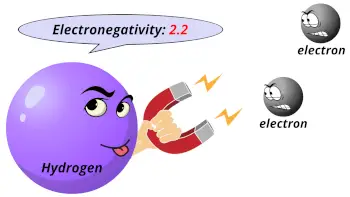 2.2 (Pauling scale) |
| Crystal structure | 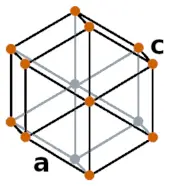 HEX (Hexagonal) |
| Melting point | 13.99 K or -259.16 °C or -434.49 °F |
| Boiling point | 20.271 K or -252.87 °C or -423.18 °F |
| Density | 0.0898 g/L |
| Main isotope | 1H, 2H |
| Who discovered Hydrogen and when? |  Henry Cavendish (In year 1766) |
| CAS number | 1333-74-0 |
Hydrogen in Periodic table
Hydrogen element is in group 1 and period 1 of the Periodic table. Hydrogen is placed along with the alkali metals group as it has the similar outer electron configuration as that of the group 1 elements (i.e ns1).
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba | La* | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| *Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| **Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
→Move to: Helium (He) element – Periodic Table
Why is Hydrogen in Group 1?
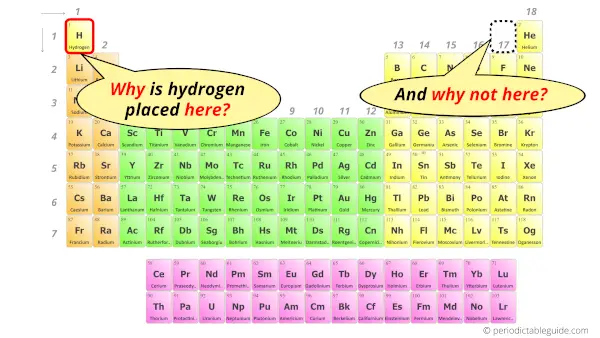
In the Periodic table, the elements having similar properties are placed together.
For example,
- Elements in group 1 have the similar properties,
- Elements in group 2 have similar properties,
- Elements in group 17 (halogens) also have similar properties.
In the same way, all the elements lying in the same group have similar properties.
Now in order to place hydrogen in Periodic table, you should know with which elements the properties of hydrogen are matching.
The interesting fact here is that, few properties of hydrogen are similar to the group 1 elements and few properties are similar to the group 17 elements.
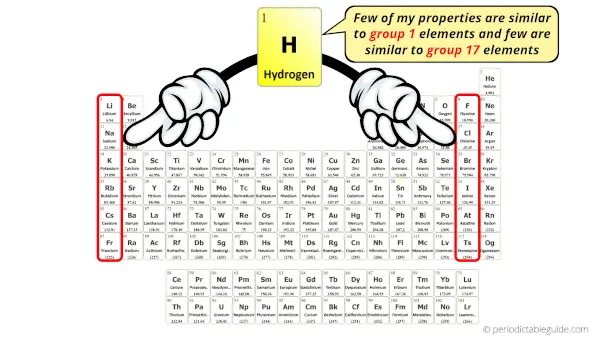
For example, group 1 elements form oxides like lithium oxide, sodium oxide, potassium oxide, etc.
The same chemical property of oxide formation is found in Hydrogen also.
Hydrogen also forms oxide (i.e H2O or dihydrogen oxide.)
Alkali metals form sulphides (like Na2S, K2S, etc) and hydrogen also forms sulphide (i.e H2S).
Alkaline metals form halides (like NaCl, KCl, etc) and hydrogen also forms halide (i.e HCl).
Apart from showing similar characteristics like that of group 1 metals, hydrogen also shows similar characteristics as that of halogens (group 17 elements).
For example halogens have very high ionization enthalpy, and hydrogen also have very high ionization enthalpy.
Also, the halogens exist as diatomic molecules (like F2, Cl2, etc). Similarly hydrogen also exists as a diatomic molecule (i.e H2).
So in short, I simply want to say that properties of hydrogen are similar to group 1 elements as well as group 17 elements.
So where should hydrogen be placed? In group 1 or group 17?
Well, in the modern printed periodic tables, you can see the Hydrogen element being placed in the group 1 along with the alkali metals.
Why?
Apart from having similar properties as that of group 1 and group 17 elements, hydrogen element is placed in 1st group because it has similar outermost electron configuration as that of alkali metals (i.e ns1).
Also see:
1). Detailed Periodic table with electron configuration (Image).
2). Electronic configuration of first 30 elements of Periodic table.
Why Hydrogen is Lightest Gas?
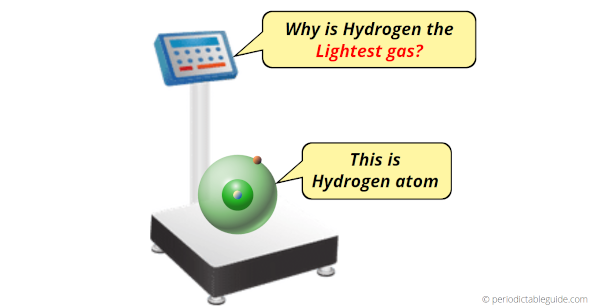
Hydrogen is the lightest gas because it consists of only one proton and one electron. Due to this, the total mass of hydrogen atom is the least of all known elements.
Explanation:
The mass of an atom completely depends on the number of protons and number of neutrons present in the nucleus of an atom.
Protons and neutrons are the heavy particles in an atom.
The mass of protons and neutrons is the same and it is 1.6749 × 10-27 kg.
While the electrons that are present in the surrounding orbits are very light.
The mass of an electron is 9.1094 × 10-31 kg, which is very negligible as compared to mass of protons and neutrons, and so it is generally not considered for calculating the atomic mass.
Now in the Hydrogen atom, there is only one proton and only one electron.
Out of all the known elements, the Hydrogen element has a least number of protons and electrons.
As hydrogen has the least number of protons and electrons, it has a least atomic mass.
In other words, atomic mass depends on the number of protons, neutrons and electrons. But hydrogen has the least number of protons and electrons, hence it has the least atomic mass.
Also see: Which is the smallest atom? Why?
Is Hydrogen Explosive? Why?

Yes, hydrogen is flammable and explosive too.
For example, if you will give fire to the pure hydrogen balloon, then it will easily catch the fire and it will burn with yellow-orange flames.
Hence hydrogen is flammable gas.
But hydrogen is explosive when it is mixed with oxygen gas.

For example, if you give fire to the balloon containing a mixture of hydrogen gas and oxygen gas, then it will blast with a loud sound like a cracker.
The mixture of hydrogen and oxygen in a proper stoichiometry ratio of 2:1 (i.e two parts of hydrogen and 1 part of oxygen) will create a very loud bursting sound.
(Note: Hydrogen cannot burn in the absence of oxygen gas. Hydrogen is a flammable gas but oxygen is necessary for producing the flame of hydrogen.
In other words, oxygen is the helper of combustion for hydrogen gas.
Without oxygen, the hydrogen cannot produce flame.)
Also see: Is helium Explosive?
How much Hydrogen is in the Air?

There is around 0.000055 % of hydrogen in the air.
Explanation:
The air consists of many gases like nitrogen, oxygen, carbon dioxide, helium, argon, neon, hydrogen, etc.
Out of all these gases, some are in abundant quantity while some are in very negligible quantity.
The hydrogen gas is in very very tiny proportion in the air.
If we talk about the total quantity occupied by the hydrogen gas, then it is only 0.000055 %.
Hydrogen gas is a very light gas (in fact it is the lightest gas).
Hence due to its light-weight, it always moves upward in the atmosphere.
Because of the light weight of hydrogen gas, it is also used in party balloons.
Is hydrogen Monatomic or Diatomic?

Hydrogen is Diatomic.
Let me explain to you the reason behind this.
Hydrogen atom has only one electron in its outermost orbit.
Now you might be knowing that having one electron in the outermost orbit is not a stable configuration.
The atom should have duplet or octet configuration for its stability.
Hydrogen has one electron in its outermost orbit and it needs one more electron to complete the duplet configuration.
Thus the hydrogen atom combines with the other hydrogen atom and completes its duplet configuration, which is stable.
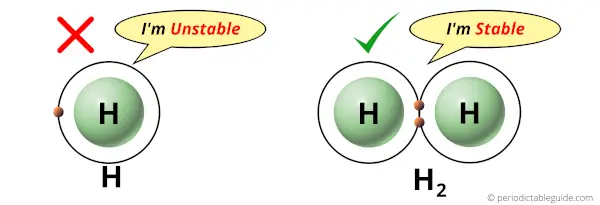
Hence, the single atom of hydrogen (H) is not stable and it always exists as a diatomic molecule (H2) which is stable.
Also read: Why is helium monatomic?
Is hydrogen soluble in water?
The solubility of hydrogen gas in water is very negligible. In other words, hydrogen is sparingly soluble in water. Solubility of hydrogen is 0.00016 grams in 100 grams of water at 1 atm and 20 °C.
Explanation:
There is one saying related to solubility that “like dissolves like”.
That means only polar solute can be dissolved in polar solvents.
But the non polar solute cannot be dissolved in the polar solvents.
Here, the hydrogen gas is non polar while water is polar.
Hence the solute hydrogen is non polar while solvent water is polar.
Hence, hydrogen is insoluble in water.
But it has been found practically that hydrogen gas shows a very very little solubility in water.
(Note: Substances like common salt, sugar, etc are polar. So they get completely dissolved in water.)
Interesting Facts about hydrogen
The interesting facts about hydrogen are mentioned below.
- Hydrogen is the only element which does not have neutrons.
- Hydrogen element is present in all living organisms. Water present in our body also contains hydrogen element. The chemical formula of water is H2O, which includes hydrogen atoms.
- It is believed that the hydrogen element was produced during the big bang.
- Hydrogen is the most abundant element found in the universe and it is roughly 74%.
- Hydrogen is the lightest gas and due to this, it escapes the earth’s atmosphere easily. Earth’s gravity cannot hold hydrogen gas in its atmosphere.
- Hydrogen gas is around 14.5 times lighter than air present around us (source).
- Hydrogen gas is found in abundance in the atmosphere of gas giant planets.
- Hydrogen is highly flammable gas, so it catches fire easily.
- All the stars are made up of hydrogen and helium gas.
Free Gift for you: Interactive Periodic Table
Let me tell you how this Interactive Periodic Table will help you in your studies.
1). You can effortlessly find every single detail about the elements from this single Interactive Periodic table.
2). You will get the detailed information about the periodic table which will convert a newbie into pro.
3). You will also get the HD images of the Periodic table (for FREE).
Checkout Interactive Periodic table and download it’s high resolution image now (It’s FREE)
External resources:
- Hydrogen – Wikipedia. (2021, December 25). Hydrogen – Wikipedia. https://en.wikipedia.org/wiki/Hydrogen
- Hydrogen – Element information, properties and uses | Periodic Table. (n.d.). Hydrogen – Element Information, Properties and Uses | Periodic Table. https://www.rsc.org/periodic-table/element/1/hydrogen
- P. (n.d.). Hydrogen | H (Element) – PubChem. Hydrogen | H (Element) – PubChem. https://pubchem.ncbi.nlm.nih.gov/element/Hydrogen
- It’s Elemental – The Element Hydrogen. (n.d.). It’s Elemental – the Element Hydrogen. https://education.jlab.org/itselemental/ele001.html
- The Chemistry of Hydrogen. (n.d.). The Chemistry of Hydrogen. https://chemed.chem.purdue.edu/genchem/topicreview/bp/ch10/hydrogen.php