This is a SUPER easy guide on Bismuth element.
In fact, the table mentioned below is the perfect information box (Which gives you every single detail about the Bismuth element in Periodic table.)
So if you want to know anything about Bismuth element, then this guide is for you.
Let’s finish this very quickly.
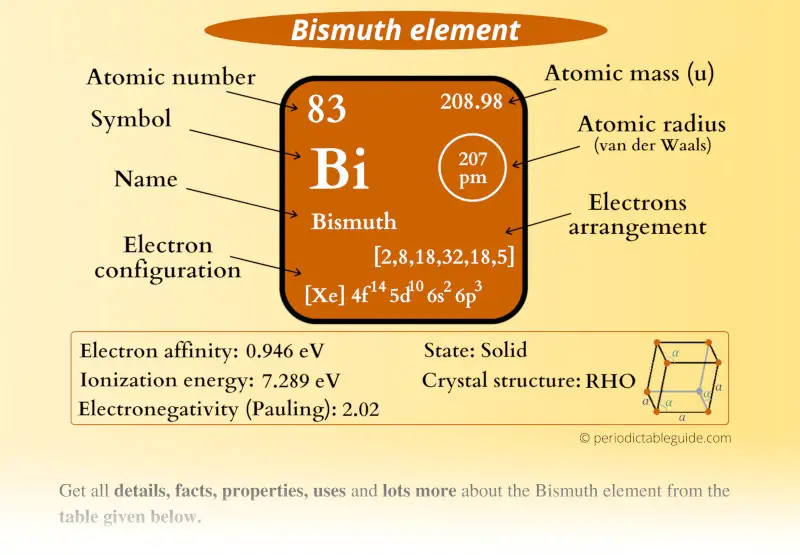
Bismuth Element (Bi) Information
| Appearance |  Silvery brown lustrous appearance |
| State (at STP) | Solid |
| Position in Periodic table | 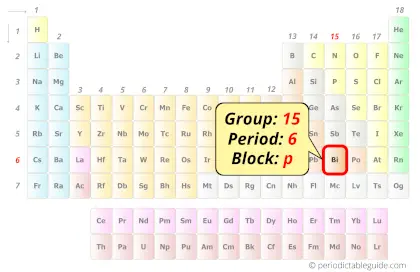 Group: 15, Period: 6, Block: p |
| Category | 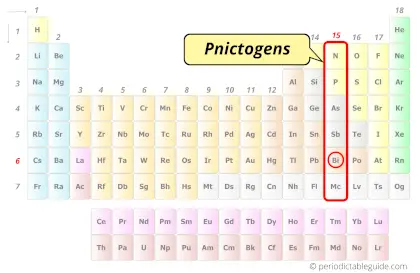 Pnictogens |
| Atomic number or Protons | 83 |
| Neutrons | 126 |
| Electrons | 83 |
| Symbol | Bi |
| Atomic mass |  208.98 u |
| Electrons arrangement or Bohr model | 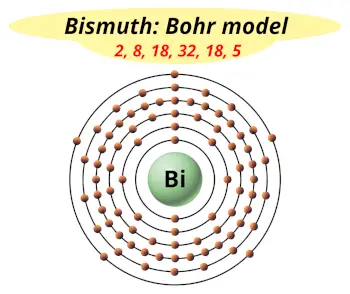 2, 8, 18, 32, 18, 5 |
| Electronic configuration | [Xe] 4f14 5d10 6s2 6p3 |
| Atomic radius | 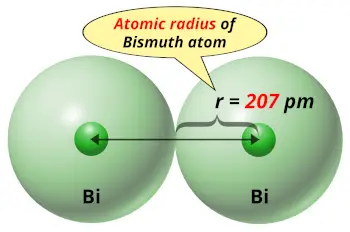 207 picometers (van der Waals radius) |
| Valence electrons | 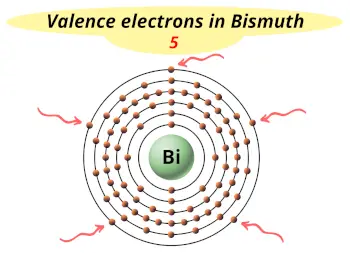 5 |
| 1st Ionization energy | 7.289 eV |
| Electronegativity | 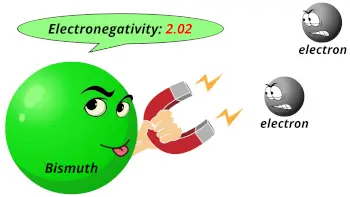 2.02 (Pauling scale) |
| Crystal structure | 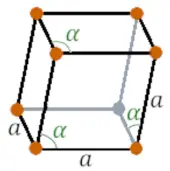 Rhombohedral |
| Melting point | 544.7 K or 271.5 °C or 520.7 °F |
| Boiling point | 1837 K or 1564 °C or 2847 °F |
| Density | 9.78 g/cm3 |
| Main isotope | 209Bi |
| CAS number | 7440-69-9 |
Bismuth in Periodic table
Bismuth element is in group 15 and period 6 of the Periodic table. Bismuth is the p-block element and it belongs to pnictogens group.
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba | La* | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| *Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| **Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
←Move to: Lead (Pb) element – Periodic Table
→Move to: Polonium (Po) element – Periodic Table
Why is Bismuth in Group 15?
Do you know, how many electrons can be accommodated in the first shell, second shell, third shell, fourth shell, etc…?
Here is the table showing the capacity of orbits to hold electrons.
Number of electrons in shells.
| Orbit / Shell (n) | Maximum no. of electrons this orbit can hold (2 × n2) |
| K shell, n = 1 | 2 × 1² = 2 |
| L shell, n = 2 | 2 × 2² = 8 |
| M shell, n = 3 | 2 × 3² = 18 |
| N shell, n = 4 | 2 × 4² = 32 |
| . . . | . . . |
Thus,
- 1st shell can hold 2 electrons.
- 2nd shell can hold 8 electrons.
- 3rd shell can hold 18 electrons.
- 4th shell can hold 32 electrons.
Now the atomic number of Bismuth (Bi) is 83.
Hence the Bismuth element has electrons arrangement 2, 8, 18, 32, 18, 5.
This electron arrangement indicates that the outermost orbit of bismuth element (Bi) has 5 electrons.
Hence, it lies in group 15.
Why is Bismuth in Period 6?
Let me ask you a question.
How many shells does bismuth have?
It’s 6. Right?
You have already seen the bohr model of bismuth atom in the above table.
From the Bohr model, it can be found that the number of orbits or shells in bismuth is 6. Hence, as bismuth has 6 orbits, it lies in period 6 of the Periodic table.
Why is Bismuth in p-block?
Before knowing this reason, first of all I want to ask you a simple question.
How can you determine the blocks-wise position of elements?
The simple answer: The elements will lie in the s, p, d or f block will completely depend upon the subshell in which the last electron will enter.
For example; the electron configuration of bismuth is [Xe] 4f14 5d10 6s2 6p3.
So the last electron of bismuth enters the p-subshell or p-orbital.
Hence, bismuth is the p-block element.
5 Interesting facts about Bismuth
Interesting facts about bismuth element are mentioned below.
- The name Bismuth came from the old German word “weissmuth”, which means “white substance”.
- The concentration of bismuth in the earth’s crust is approximately 9 parts per billion by weight.
- The majority of bismuth is obtained from its mineral ore Bismuth sulfide.
- Bismuth is one of the few elements that expands on freezing. In other words, bismuth is more dense in liquid phase than in solid phase. Other elements that expand on freezing are gallium, antimony, silicon and germanium.
- The annual production of bismuth in the entire world is approximately 9000 tones.
Properties of Bismuth
The physical and chemical properties of bismuth element are mentioned below.
Physical properties of Bismuth
Physical properties of bismuth are mentioned below.
- Bismuth is a crystalline brittle metal that has a silvery brown lustrous appearance.
- Bismuth is a diamagnetic metal and it is repelled by the magnetic field.
- Bismuth is a poor conductor of heat and electricity.
- The melting point and boiling point of bismuth are 271.5 °C and 1564 °C respectively.
- Bismuth has many isotopes. Out of those isotopes, the most abundant isotope is 209Bi (which has an abundance of almost 100%).
- The crystal structure of bismuth is Rhombohedral.
Chemical properties of Bismuth
Chemical properties of bismuth are mentioned below.
- At room temperature, bismuth is stable and does not react with dry or moist air.
- At higher temperature, bismuth reacts with water and forms bismuth (III) oxide.
- Bismuth also reacts with halogens like fluorine, chlorine, bromine, etc to form bismuth (III) halides.
- Bismuth dissolves in concentrated sulfuric acid as well as hydrochloric acid.
- The 1st ionization energy of bismuth is 7.289 eV.
- The electronegativity of bismuth is 2.02 on the Pauling scale.
Uses of Bismuth
Uses of bismuth are mentioned below.
- As bismuth has a lower melting point, it is used in making low melting alloys.
- Bismuth has many applications in pharmaceutical industries, for making medicines.
- Bismuth is also used in cosmetics as well as pigments.
- The compounds of bismuth are used as a catalyst in making acrylic fibers.
- Bismuth compounds are also used in making grease for lubrication.
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
External resources:
- Bismuth – Element information, properties and uses | Periodic Table. (n.d.). Bismuth – Element Information, Properties and Uses | Periodic Table. https://www.rsc.org/periodic-table/element/83/bismuth
- Bismuth – Wikipedia. (2020, April 7). Bismuth – Wikipedia. https://en.wikipedia.org/wiki/Bismuth
- P. (n.d.). Bismuth | Bi (Element) – PubChem. Bismuth | Bi (Element) – PubChem. https://pubchem.ncbi.nlm.nih.gov/element/Bismuth
- It’s Elemental – The Element Bismuth. (n.d.). It’s Elemental – the Element Bismuth. https://education.jlab.org/itselemental/ele083.html
- Bismuth. (n.d.). Bismuth. https://nature.berkeley.edu/classes/eps2/wisc/bismuth.html
- Kanatzidis, M., Sun, H., & Dehnen, S. (2020, March 16). Bismuth—The Magic Element. Inorganic Chemistry, 59(6), 3341–3343. https://doi.org/10.1021/acs.inorgchem.0c00222
