This is a SUPER easy guide on Polonium element.
In fact, the table mentioned below is the perfect information box (Which gives you every single detail about the Polonium element in Periodic table.)
So if you want to know anything about the Polonium element, then this guide is for you.
Let’s dive right into it!
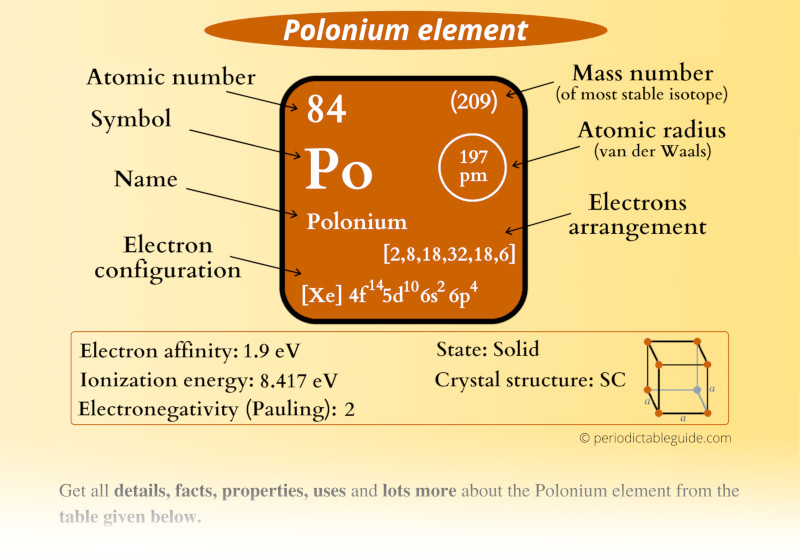
Polonium Element (Po) Information
| Appearance | Silvery-grey |
| State (at STP) | Solid |
| Position in Periodic table | 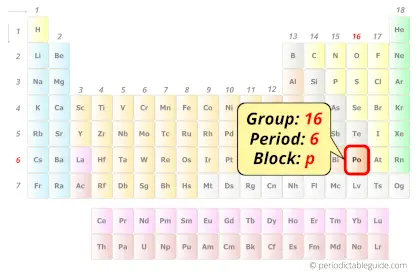 Group: 16, Period: 6, Block: p |
| Category | 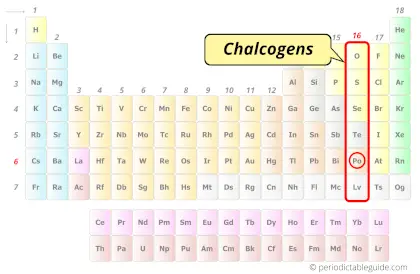 Chalcogens |
| Atomic number or Protons | 84 |
| Neutrons | 125 |
| Electrons | 84 |
| Symbol | Po |
| Atomic mass of Polonium (most stable isotope) | 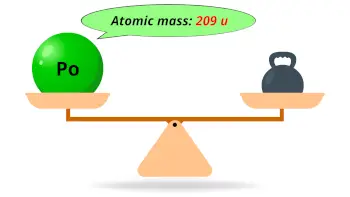 209 u |
| Electrons arrangement or Bohr model | 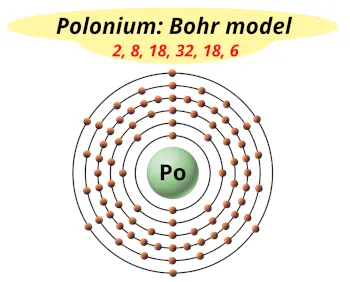 2, 8, 18, 32, 18, 6 |
| Electronic configuration | [Xe] 4f14 5d10 6s2 6p4 |
| Atomic radius | 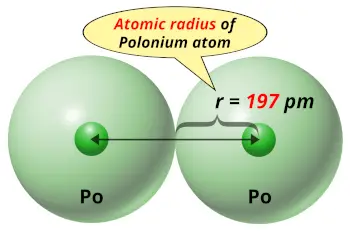 197 picometers (van der Waals radius) |
| Valence electrons | 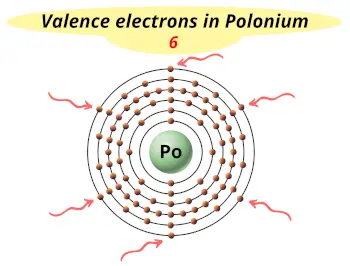 6 |
| 1st Ionization energy | 8.417 eV |
| Electronegativity | 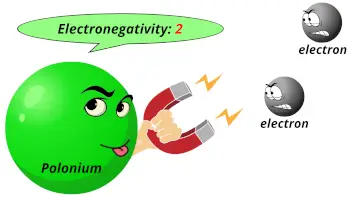 2 (Pauling scale) |
| Crystal structure | 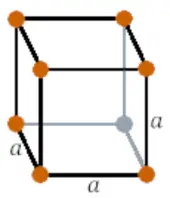 SC (Simple cubic) |
| Melting point | 527 K or 254 °C or 489 °F |
| Boiling point | 1235 K or 962 °C or 1764 °F |
| Density | 9.16 g/cm3 |
| Main isotope | 210Po |
| Who discovered Polonium and when? |  Pierre Curie and Marie Curie (in 1898) |
| CAS number | 7440-08-6 |
Polonium in Periodic table
Polonium element is in group 16 and period 6 of the Periodic table. Polonium is the p-block element and it belongs to chalcogens group.
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba | La* | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| *Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| **Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
←Move to: Bismuth (Bi) element – Periodic Table
→Move to: Astatine (At) element – Periodic Table
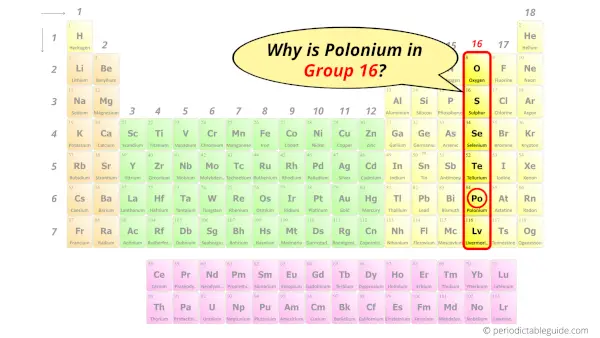
Why is Polonium in Group 16?
Do you know, how many electrons can be accommodated in the first shell, second shell, third shell, fourth shell, etc…?
Here is the table showing the capacity of orbits to hold electrons.
Number of electrons in shells.
| Orbit / Shell (n) | Maximum no. of electrons this orbit can hold (2 × n2) |
| K shell, n = 1 | 2 × 1² = 2 |
| L shell, n = 2 | 2 × 2² = 8 |
| M shell, n = 3 | 2 × 3² = 18 |
| N shell, n = 4 | 2 × 4² = 32 |
| . . . | . . . |
Thus,
- 1st shell can hold 2 electrons.
- 2nd shell can hold 8 electrons.
- 3rd shell can hold 18 electrons.
- 4th shell can hold 32 electrons.
Now the atomic number of Polonium (Po) is 84.
Hence the polonium element has electrons arrangement 2, 8, 18, 32, 18, 6.
This electron arrangement indicates that the outermost orbit of polonium element (Po) has 6 electrons.
Hence, it lies in group 16.
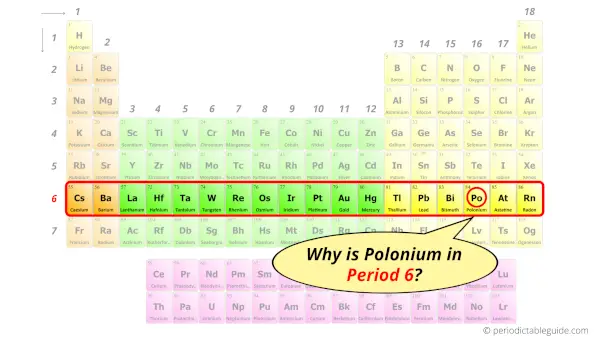
Why is Polonium in Period 6?
Let me ask you a question.
How many shells does polonium have?
It’s 6. Right?
You have already seen the bohr model of polonium atom in the above table.
From the Bohr model, it can be found that the number of orbits or shells in polonium is 6. Hence, as polonium has 6 orbits, it lies in period 6 of the Periodic table.
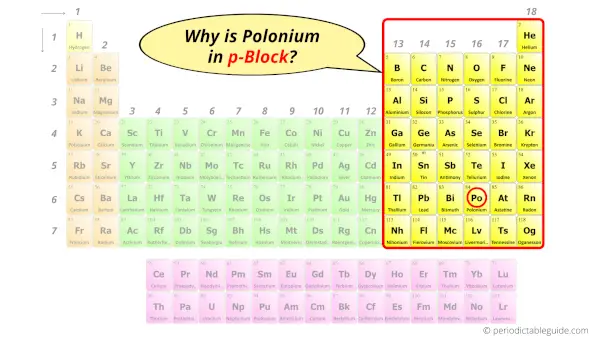
Why is Polonium in p-block?
Before knowing this reason, first of all I want to ask you a simple question.
How can you determine the blocks-wise position of elements?
The simple answer: The elements will lie in the s, p, d or f block will completely depend upon the subshell in which the last electron will enter.
For example; the electron configuration of polonium is [Xe] 4f14 5d10 6s2 6p4.
So the last electron of polonium enters the p-subshell or p-orbital.
Hence, polonium is the p-block element.
6 Interesting facts about Polonium
Interesting facts about polonium element are mentioned below.
- Polonium was discovered by Pierre Curie and Marie Curie in 1898.
- The name polonium was named from the country “Poland”, where Marie curie was born and grew up.
- Polonium is extremely rare in the earth’s crust. Its concentration in the earth’s crust is around 1 part per quadrillion.
- Polonium is so radioactive that 1 gram of polonium can emit as many alpha particles as 5 kilogram of radium.
- According to IUPAC, the polonium element is 400 times more radioactive than uranium.
- Polonium is generally found in the uranium ores. Only 100 micrograms of polonium can be obtained from 1 ton of uranium ore.
Properties of Polonium
The physical and chemical properties of polonium element are mentioned below.
Physical properties of Polonium
Physical properties of polonium are mentioned below.
- Polonium is a solid metal having a Silvery-grey appearance.
- Polonium is one of the few metals that have a lower melting point. The melting point of polonium is 254 °C and its boiling point is 962 °C.
- Polonium has many isotopes, and all those isotopes are radioactive. The isotope 210Po is available in very trace amounts and the rest of the isotopes are mostly synthetically prepared in the lab.
- The atomic mass of the most stable isotope of polonium is 209 u and its density is 9.16 g/cm3.
Chemical properties of Polonium
Chemical properties of polonium are mentioned below.
- Polonium is a chemically toxic and radioactive element.
- Polonium easily gets dissolved in dilute acids.
- In alkalis, polonium is slightly soluble.
- The polonium solutions are initially pink in color due to the presence of Po2+ ions, but they rapidly become yellow due to the emissions of alpha radiations that convert Po2+ into Po4+ ions.
Uses of Polonium
Uses of polonium are mentioned below.
- 1 gram of Polonium-210 can heat upto 500 °C, hence it is used as a heat source in radioisotope thermoelectric generators.
- Polonium is also used in anti-static brushes for eliminating the dust on photographic film.
- When polonium is alloyed with beryllium, it is used as a neutron source.
- The polonium element can also be used in eliminating static electricity during the sheet metal rolling process.
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
External resources:
- Polonium – Element information, properties and uses | Periodic Table. (n.d.). Polonium – Element Information, Properties and Uses | Periodic Table. https://www.rsc.org/periodic-table/element/84/polonium
- Polonium – Wikipedia. (2014, September 9). Polonium – Wikipedia. https://en.wikipedia.org/wiki/Polonium
- P. (n.d.). Polonium | Po (Element) – PubChem. Polonium | Po (Element) – PubChem. https://pubchem.ncbi.nlm.nih.gov/element/Polonium
- It’s Elemental – The Element Polonium. (n.d.). It’s Elemental – the Element Polonium. https://education.jlab.org/itselemental/ele084.html
- Radiation Studies: CDC – Radiation: Polonium-210 | CDC RSB. (2014, January 7). Radiation Studies: CDC – Radiation: Polonium-210 | CDC RSB. https://www.cdc.gov/nceh/radiation/polonium-210.htm
- Atomic Data for Polonium (Po). (n.d.). Atomic Data for Polonium (Po). https://physics.nist.gov/PhysRefData/Handbook/Tables/poloniumtable1.htm
- C&EN: IT’S ELEMENTAL: THE PERIODIC TABLE – POLONIUM. (n.d.). C&EN: IT’S ELEMENTAL: THE PERIODIC TABLE – POLONIUM. https://pubsapp.acs.org/cen/80th/polonium.html?
- Ansoborlo, E. (2014, April 22). Poisonous polonium. Nature Chemistry, 6(5), 454–454. https://doi.org/10.1038/nchem.1928
