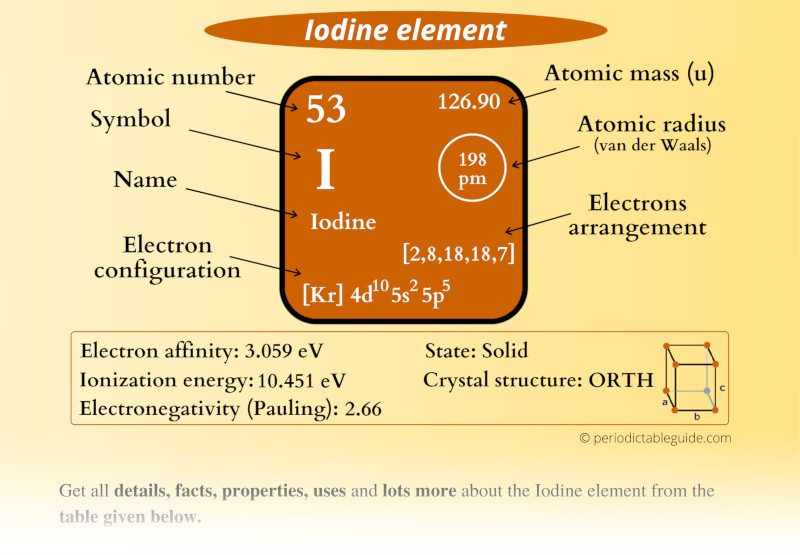
This is a SUPER easy guide on Iodine element.
In fact, the table mentioned below is the perfect information box (Which gives you every single detail about the Iodine element in Periodic table.)
So if you want to know anything about Iodine element, then this guide is for you.
Let’s dive right into it!
Iodine Element (I) Information
| Appearance |  Solid: Lustrous metallic gray Liquid: Violet colored liquid Gas: Violet gas |
| State (at STP) | Solid |
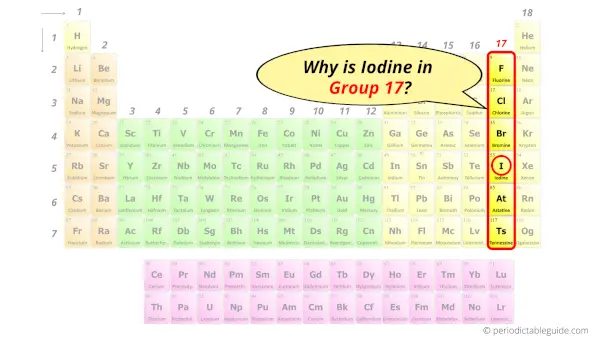
| Position in Periodic table | 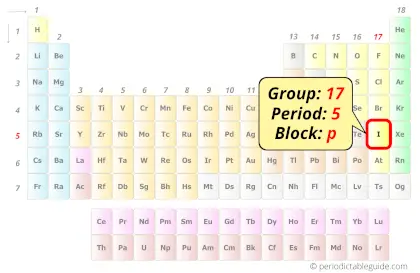 Group: 17, Period: 5, Block: p |
| Category | 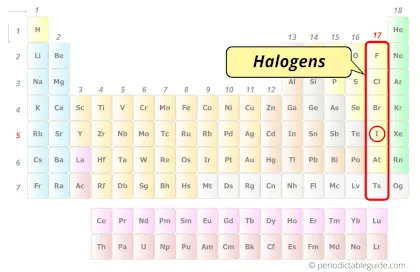 Halogens |
| Atomic number or Protons | 53 |
| Neutrons | 74 |
| Electrons | 53 |
| Symbol | I |
| Atomic mass |  126.90 u |
| Electrons arrangement or Bohr model | 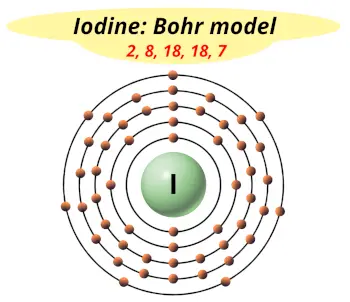 2, 8, 18, 18, 7 |
| Electronic configuration | [Kr] 4d10 5s2 5p5 |
| Atomic radius | 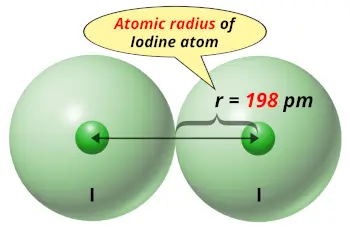 198 picometers (van der Waals radius) |
| Valence electrons | 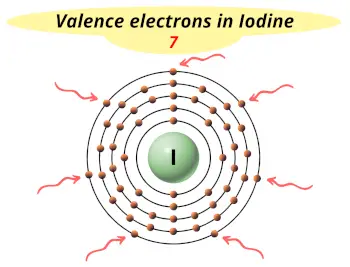 7 |
| 1st Ionization energy | 10.451 eV |
| Electronegativity | 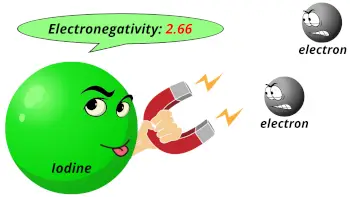 2.66 (Pauling scale) |
| Crystal structure | 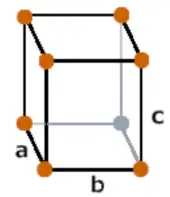 Orthorhombic |
| Melting point | 386.8 K or 113.7 °C or 236.6 °F |
| Boiling point | 457.4 K or 184.3 °C or 363.7 °F |
| Density | 4.94 g/cm3 |
| Main isotope | 127I |
| Who discovered Iodine and when? | Bernard Courtois in 1811 |
| CAS number | 7553-56-2 |
Iodine in Periodic table
Iodine element is in group 17 and period 5 of the Periodic table. Iodine is the p-block element and it belongs to halogens group.
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba | La* | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| *Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| **Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
←Move to: Tellurium (Te) element – Periodic Table
→Move to: Xenon (Xe) element – Periodic Table
Why is Iodine in Group 17?
Do you know, how many electrons can be accommodated in the first shell, second shell, third shell, fourth shell, etc…?
Here is the table showing the capacity of orbits to hold electrons.
Number of electrons in shells.
| Orbit / Shell (n) | Maximum no. of electrons this orbit can hold (2 × n2) |
| K shell, n = 1 | 2 × 1² = 2 |
| L shell, n = 2 | 2 × 2² = 8 |
| M shell, n = 3 | 2 × 3² = 18 |
| N shell, n = 4 | 2 × 4² = 32 |
| . . . | . . . |
Thus,
- 1st shell can hold 2 electrons.
- 2nd shell can hold 8 electrons.
- 3rd shell can hold 18 electrons.
- 4th shell can hold 32 electrons, and so on.
Now the atomic number of iodine (I) is 53.
Hence the electron arrangement in iodine is 2, 8, 18, 18, 7. And the electron configuration of iodine is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p5.
This electron arrangement and electron configuration indicates that the outermost orbit (i.e orbit number 5) of an iodine atom has 7 electrons.
Hence, it lies in group 17.
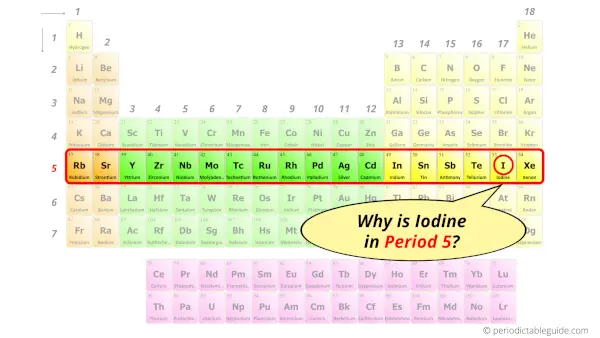
Why is Iodine in Period 5?
Let me ask you a question.
How many shells does iodine have?
It’s 5. Right?
You have already seen the bohr model of iodine atom in the above table.
From the Bohr model, it can be found that the number of orbits or shells in iodine is 5. Hence, as iodine has 5 orbits, it lies in period 5 of the Periodic table.
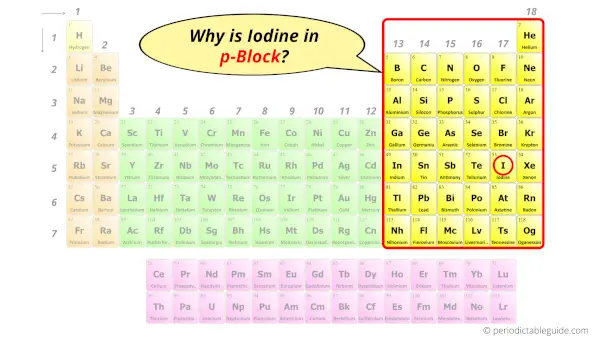
Why is Iodine in p-block?
Before knowing this reason, first of all I want to ask you a simple question.
How can you determine the blocks-wise position of elements?
The simple answer: The elements will lie in the s, p, d or f block will completely depend upon the subshell in which the last electron will enter.
For example; the electron configuration of iodine is [Kr] 4d10 5s2 5p5.
So the last electron of iodine enters the p-subshell or p-orbital.
Hence, iodine is the p-block element.
7 Interesting facts about Iodine
Interesting facts about iodine element are mentioned below.
- The name iodine was derived from the Greek word “iodes”, which means violet color.
- Iodine is the 61st abundant element present on the earth, which is considered to be a rarest element needed for life.
- Iodine is the 47th abundant element in the solar system.
- Iodine is a very rare element, but it is found in very trace amounts in our surroundings (including soil, plants, water, animals, humans, etc.)
- Sea water contains around 34.5 million tons of iodine, and this makes it the largest reservoir of iodine on the earth.
- Sea water contains around 50 to 60 parts per billion of iodine, while river water contains around 5 parts per billion of iodine.
- Iodine was discovered and first isolated by Bernard Courtois in 1811.
Properties of Iodine
The physical and chemical properties of iodine element are mentioned below.
Physical properties of Iodine
Physical properties of iodine are mentioned below.
- The pure iodine is solid at STP and it is lustrous metallic gray in color.
- The melting point of iodine (I2) is 113.7 °C and its boiling point is 184.3 °C.
- The atomic mass of iodine is 126.90 u and its density is 4.94 g/cm3.
- Iodine has an Orthorhombic crystal structure.
- There are many isotopes of iodine, but only one is stable (and that is 127I). Rest of the isotopes are synthetic isotopes which are artificially prepared in the lab.
Chemical properties of Iodine
Chemical properties of iodine are mentioned below.
- Iodine is a chemically reactive nonmetal and hence it is not found in a free state in nature but it exists as a compound with other elements.
- When iodine reacts with metals, it forms salts.
- Iodine is the least electronegative element out of other halogens present above it in group 17. The electronegativity of iodine is 2.66 on the Pauling scale.
- The solubility of iodine in water is less, but its solubility in nonpolar solvents is more.
- Pure iodine is dangerous and it can even burn the skin.
Uses of Iodine
Uses of iodine are mentioned below.
- The table salt which we use in our food contains iodine ions in trace amounts which fulfills the need of iodine in our diet. (Such salts are called iodized salts).
- Iodine is also used in antiseptics to kill bacteria and germs.
- The cancer of thyroid gland can be treated by using a radioactive isotope of iodine (131I).
- In chemical industries, Iodine is used in production of acetic acid.
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
External resources:
- Iodine – Wikipedia. (2017, November 20). Iodine – Wikipedia. https://en.wikipedia.org/wiki/Iodine
- Iodine – Element information, properties and uses | Periodic Table. (n.d.). Iodine – Element Information, Properties and Uses | Periodic Table. https://www.rsc.org/periodic-table/element/53/iodine
- P. (n.d.). Iodine | I (Element) – PubChem. Iodine | I (Element) – PubChem. https://pubchem.ncbi.nlm.nih.gov/element/Iodine
- C&EN: IT’S ELEMENTAL: THE PERIODIC TABLE – IODINE. (n.d.). C&EN: IT’S ELEMENTAL: THE PERIODIC TABLE – IODINE. https://pubsapp.acs.org/cen/80th/iodine.html?
- It’s Elemental – The Element Iodine. (n.d.). It’s Elemental – the Element Iodine. https://education.jlab.org/itselemental/ele053.html
