This is a SUPER easy guide on Tennessine element.
In fact, the table mentioned below is the perfect information box (Which gives you every single detail about the Tennessine element in Periodic table.)
So if you want to know anything about Tennessine element, then this guide is for you.
Let’s dive right into it!
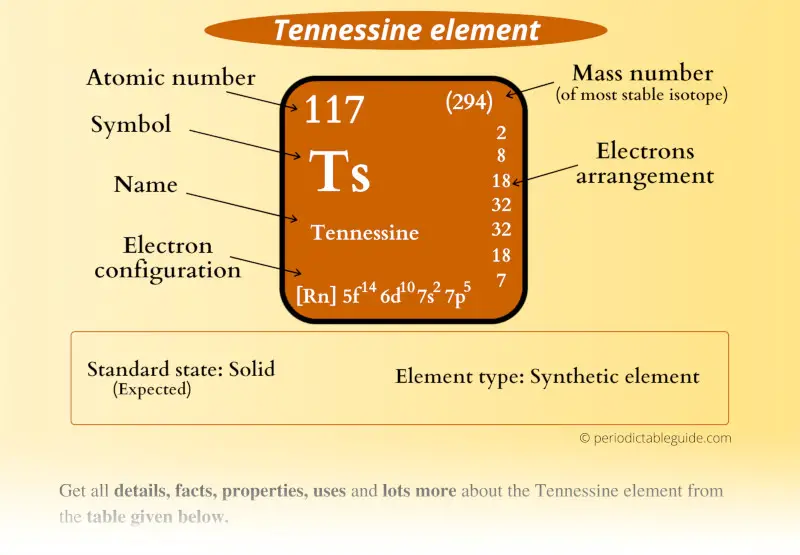
Tennessine Element (Ts) Information
| Appearance | Semi Metallic (predicted) |
| State (at STP) | Solid (predicted) |
| Position in Periodic table | 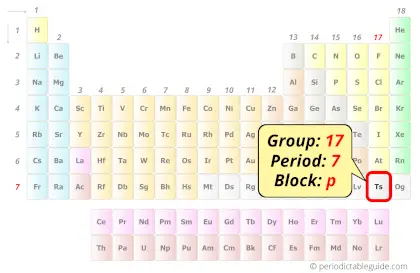 Group: 17, Period: 7, Block: p |
| Category | Synthetic element |
| Atomic number or Protons | 117 |
| Electrons | 117 |
| Symbol | Ts |
| Atomic mass of Tennessine (most stable isotope) | 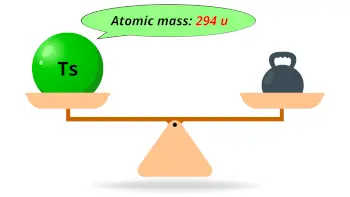 294 u |
| Electrons arrangement or Bohr model | 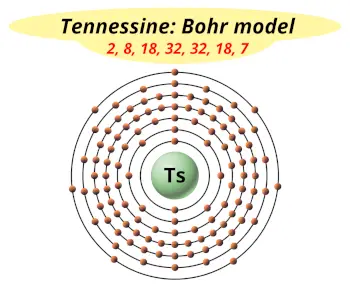 2, 8, 18, 32, 32, 18, 7 |
| Electronic configuration | [Rn] 5f14 6d10 7s2 7p5 |
| Density (predicted) | 7.1-7.3 g/cm3 |
| Main isotope | 294Ts |
| CAS number | 54101-14-3 |
Tennessine in Periodic table
Tennessine element is in group 17 and period 7 of the Periodic table. Tennessine is the p-block element and it is a radioactive synthetic element.
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba | La* | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| *Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| **Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
←Move to: Livermorium (Lv) element – Periodic Table
→Move to: Oganesson (Og) element – Periodic Table
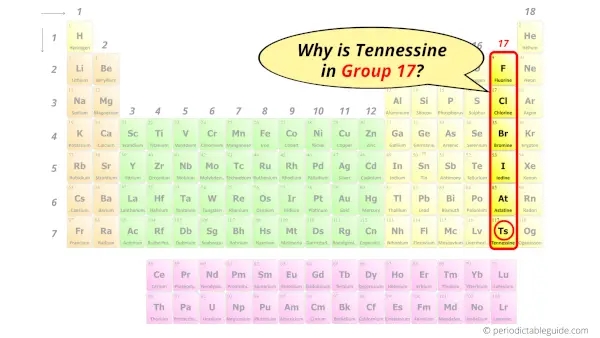
Why is Tennessine in Group 17?
Do you know, how many electrons can be accommodated in the first shell, second shell, third shell, fourth shell, etc…?
Here is the table showing the capacity of orbits to hold electrons.
Number of electrons in shells.
| Orbit / Shell (n) | Maximum no. of electrons this orbit can hold (2 × n2) |
| K shell, n = 1 | 2 × 1² = 2 |
| L shell, n = 2 | 2 × 2² = 8 |
| M shell, n = 3 | 2 × 3² = 18 |
| N shell, n = 4 | 2 × 4² = 32 |
| . . . | . . . |
Thus,
- 1st shell can hold 2 electrons.
- 2nd shell can hold 8 electrons.
- 3rd shell can hold 18 electrons.
- 4th shell can hold 32 electrons, and so on…
Now the atomic number of Tennessine (Ts) is 117.
Hence the Tennessine element has electrons arrangement 2, 8, 18, 32, 32, 18, 7.
This electron arrangement indicates that the outermost orbit of tennessine element (Ts) has 7 electrons.
Hence, it lies in group 17.
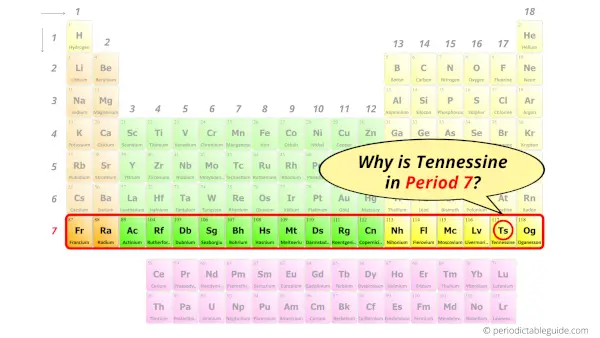
Why is Tennessine in Period 7?
Let me ask you a question.
How many shells does a tennessine atom have?
It’s 7. Right?
You have already seen the bohr model of tennessine atom in the above table.
From the Bohr model, it can be found that the number of orbits or shells in tennessine is 7. Hence, as tennessine has 7 orbits, it lies in period 7 of the Periodic table.
5 facts about Tennessine
Interesting facts about tennessine element are mentioned below.
- Tennessine was given its name from the “Tennessee”, which is a state in the southeast of the United States.
- Tennessine is extremely radioactive and it is not available naturally. It is artificially prepared in a laboratory.
- Tennessine was discovered by the researchers at Lawrence Livermore National laboratory (California), Joint Institute for Nuclear Research (Russia), and Oak Ridge National Laboratory (Tennessee).
- All the isotopes of tennessine are radioactive in nature.
- Out of all the known isotopes of tennessine, the longest lived isotope is 294Ts, with a half life of only 51 milliseconds.
Properties of Tennessine
The physical and chemical properties of tennessine element are mentioned below.
- Tennessine is highly radioactive and it has a very short half life (in milliseconds).
- The tennessine element is predicted to have a semi metallic appearance and it is predicted to have solid phase at room temperature.
- The estimated atomic mass of the most stable isotope is tennessine is 294 u and its estimated density is 7.1-7.3 g/cm3.
- Tennessine is predicted to have +1 and +3 common oxidation states.
Uses of Tennessine
Tennessine is basically used for scientific research work. Tennessine has no commercial use due to its high radioactivity and its expensive production.
Free Gift for you: Interactive Periodic Table
Let me tell you how this Interactive Periodic Table will help you in your studies.
1). You can effortlessly find every single detail about the elements from this single Interactive Periodic table.
2). You will get the detailed information about the periodic table which will convert a newbie into pro.
3). You will also get the HD images of the Periodic table (for FREE).
Checkout Interactive Periodic table and download it’s high resolution image now (It’s FREE)
External resources:
- Tennessine – Wikipedia. (2017, December 19). Tennessine – Wikipedia. https://en.wikipedia.org/wiki/Tennessine
- Tennessine – Element information, properties and uses | Periodic Table. (n.d.). Tennessine – Element Information, Properties and Uses | Periodic Table. https://www.rsc.org/periodic-table/element/117/tennessine
- P. (n.d.). Tennessine | Ts (Element) – PubChem. Tennessine | Ts (Element) – PubChem. https://pubchem.ncbi.nlm.nih.gov/element/Tennessine
- It’s Elemental – The Element Tennessine. (n.d.). It’s Elemental – the Element Tennessine. https://education.jlab.org/itselemental/ele117.html
- Element 117 timeline | ORNL. (n.d.). Element 117 Timeline | ORNL. https://www.ornl.gov/content/element-117-timeline