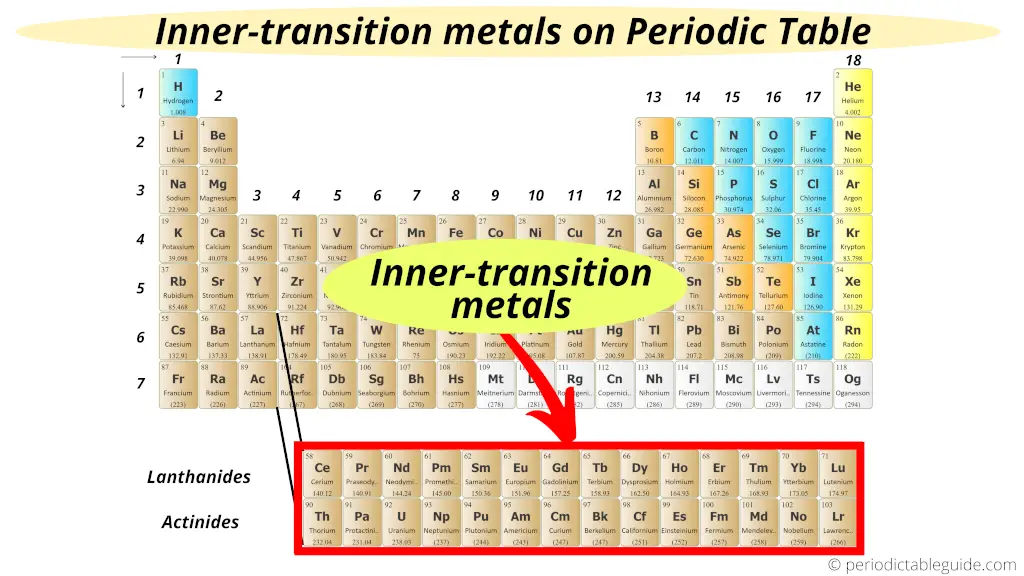
Inner transition metals are located in the two rows at the bottom of the periodic table.
From above image, you can easily find where Inner Transition Metals are located on Periodic Table.
These two rows at the bottom of the Periodic table are called;
- Lanthanides (Ce – Lu, having atomic number from 58 – 71) and
- Actinides (Th – Lr, having atomic number from 90 – 103)
But wait… A question for you.
Do you know why are inner transition metals at the bottom of the periodic table?
Don’t worry, you will get your answer in just a few seconds.
Here I have covered these important topics;
- Why inner transition elements are called so?
- Why are inner transition metals at the bottom of the periodic table?
- Inner transition metals list with Electronic configuration
- Inner transition metals facts
- Inner transition metals uses
- Difference between transition and inner transition elements
Let’s finish this very quickly.
Why Inner Transition Elements are called so?
The short answer:
Because they have similar properties like transition elements, and they are placed in the inner section as an extension of group 3.
Let me explain this in short.
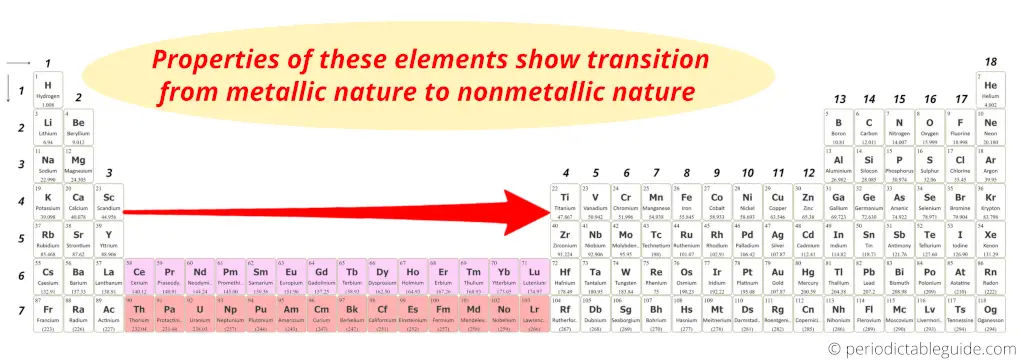
You can see in the above image that these elements have the properties similar to that of transition elements.
Plus
They are placed in the modern Periodic table as the inner part of group 3.
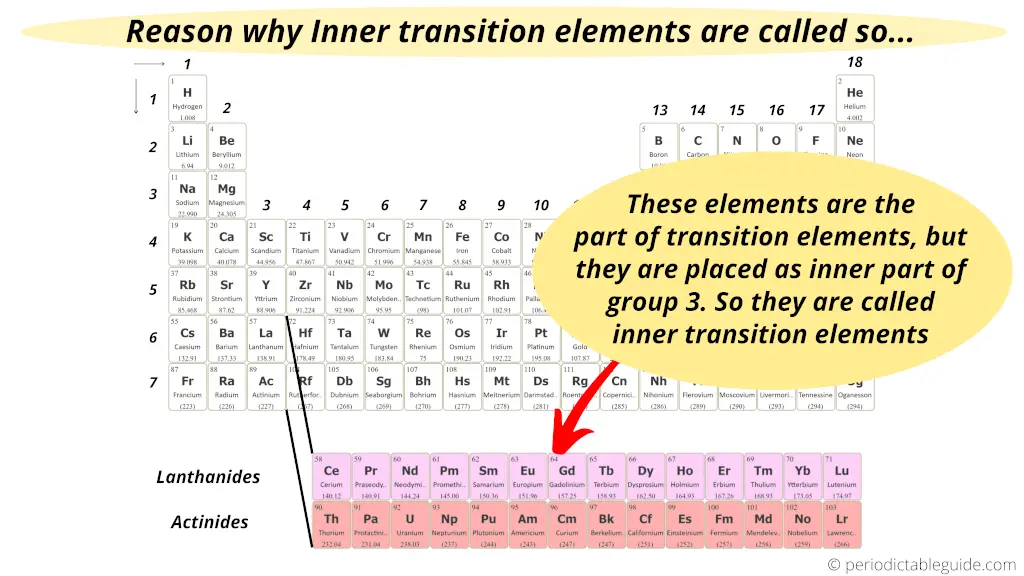
As a summary, just remember these two points;
- The properties of these elements show the transition from electropositive nature to electronegative nature.
- Also they are in the inner section of the periodic table, like an extension of the group 3 elements.
Hence these elements are called inner transition elements.
Why are inner transition metals at the bottom of the periodic table?
Just think, what if they were not placed at the bottom?
The simple answer: Periodic table would look something like this (see below image).
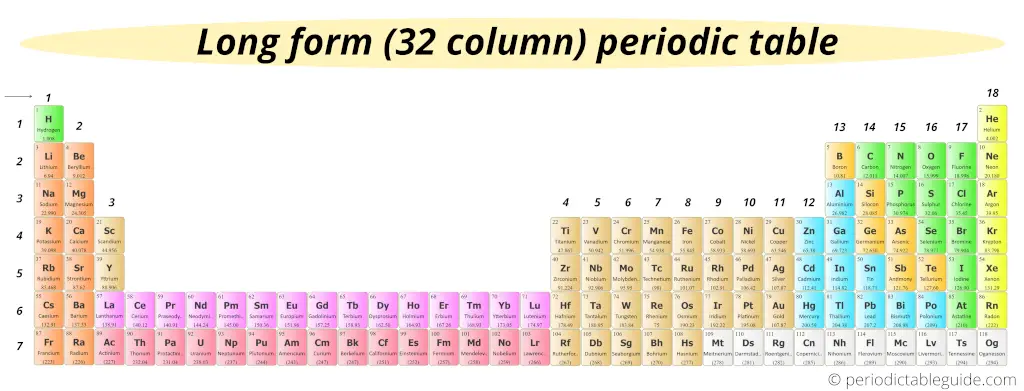
It’s very long. Right?
If the inner transition elements were not placed in the bottom, then there will be a longer periodic table like this.
So to make the Periodic table short and fit perfectly on A4 size paper, the two rows (lanthanoids and actinoids) are placed at the bottom of the Periodic table.
Plus
The elements lying in these two rows also possess similar properties.
They have their valence electrons in the f-orbitals. Hence, the transition metals (lanthanoids and actinoids) are placed separately in the two rows at the bottom of the Periodic table.
And as inner transition elements have valence electrons in f-orbitals, they are also known as f-block elements.
Inner transition metals list with electronic configuration
The list of inner transition metals with atomic number, symbol, name of element and electron configuration is given below.
| Atomic number | Symbol | Name of element | Electronic configuration |
| 58 | Ce | Cerium | [Xe] 4f1 5d1 6s2 |
| 59 | Pr | Praseodymium | [Xe] 4f3 6s2 |
| 60 | Nd | Neodymium | [Xe] 4f4 6s2 |
| 61 | Pm | Promethium | [Xe] 4f5 6s2 |
| 62 | Sm | Samarium | [Xe] 4f6 6s2 |
| 63 | Eu | Europium | [Xe] 4f7 6s2 |
| 64 | Gd | Gadolinium | [Xe] 4f7 5d1 6s2 |
| 65 | Tb | Terbium | [Xe] 4f9 6s2 |
| 66 | Dy | Dysprosium | [Xe] 4f10 6s2 |
| 67 | Ho | Holmium | [Xe] 4f11 6s2 |
| 68 | Er | Erbium | [Xe] 4f12 6s2 |
| 69 | Tm | Thulium | [Xe] 4f13 6s2 |
| 70 | Yb | Ytterbium | [Xe] 4f14 6s2 |
| 71 | Lu | Lutetium | [Xe] 4f14 5d1 6s2 |
| 90 | Th | Thorium | [Rn] 6d2 7s2 |
| 91 | Pa | Protactinium | [Rn] 5f2 6d1 7s2 |
| 92 | U | Uranium | [Rn] 5f3 6d1 7s2 |
| 93 | Np | Neptunium | [Rn] 5f4 6d1 7s2 |
| 94 | Pu | Plutonium | [Rn] 5f6 7s2 |
| 95 | Am | Americium | [Rn] 5f7 7s2 |
| 96 | Cm | Curium | [Rn] 5f7 6d1 7s2 |
| 97 | Bk | Berkelium | [Rn] 5f9 7s2 |
| 98 | Cf | Californium | [Rn] 5f10 7s2 |
| 99 | Es | Einsteinium | [Rn] 5f11 7s2 |
| 100 | Fm | Fermium | [Rn] 5f12 7s2 |
| 101 | Md | Mendelevium | [Rn] 5f13 7s2 |
| 102 | No | Nobelium | [Rn] 5f14 7s2 |
| 103 | Lr | Lawrencium | [Rn] 5f14 7s2 7p1 |
Also visit: List of transition metals with their electron configuration
Inner transition metals facts
The facts of inner transition metals are mentioned below.
- The lanthanides are found naturally from the earth crust but they are found from very rare locations.
- Most actinides elements are artificially prepared in laboratory and they are radioactive in nature.
- The inner transition elements have the valence electrons in the f-orbitals.
- Some lanthanides are soft and can be cut with a knife.
- The actinides elements of inner transition metals are strong reducing agents.
Inner transition metals uses
The uses of inner transition metals are mentioned below.
- Uranium and plutonium are inner transition metals which are used for manufacturing nuclear weapons.
- Radioactive metals like uranium produce a lot of heat when they undergo nuclear fission. This heat is used to produce the steam from the water. Hence uranium is also used in nuclear power plants.
- Neodymium (Nd), Cerium (Cm) and Samarium (Sm) are mixed with other metals to prepare strong magnets.
- They are also used in manufacturing the lense of sunglasses, which protects our eyes from UV rays.
- Lanthanoids are mostly used in manufacturing lazer.
Difference between transition and inner transition elements
The difference between transition and inner transition elements are given below.
| No. | Transition elements | Inner transition elements |
|---|---|---|
| 1 | Transition elements have valence electrons in d-orbitals. | Inner transition elements have valence electrons in f-orbitals. |
| 2 | Transition elements lie in the middle part of the periodic table. | Inner transition elements are located at the bottom of the periodic table. |
| 3 | Mostly transition metals are obtained from the earth crust in the form of compounds. | Inner transition metals are mostly synthetic radioactive elements. Lanthanides are mostly obtained from the earth crust while actinides are artificially prepared in a laboratory. |
| 4 | Transition metals have higher density and are hard and tough. | Some of the lanthanides are soft and can be cut with a kitchen knife. |
| 5 | The general education configuration of transition metals or d-block elements is (n-1)d(1-10) ns(0-2) | The general education configuration of inner transition metals or f-block elements is (n-2)f(0-14) (n-1)d(0-1) ns2 |
Also Read:
1). Where are and how many transition metals are on periodic table?
2). Where are Halogens on periodic table?
3). Where are Alkali metals on periodic table?
4). Where are Alkaline earth metals on periodic table?
5). Where are Noble gases on periodic table?
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
Summary
So in the very beginning, I showed you a single image that clarifies you where are inner transition metals located on Periodic table.
They are placed in the separate two rows below the Periodic table known as Lanthanides and Actinides and they are also known as f-block elements.
Then we saw the reason why they are called inner transition metals?
Also I have told you the reason why lanthanoids and actinoids are placed at the bottom of the Periodic table.
Later on, we saw the complete list of inner transition metals along with their electron configuration.
The facts and uses of inner transition elements are just amazing. Hope now you know some real life application of such elements.
Lastly we saw the difference between transition and inner transition elements.
I hope this article has helped you in solving your doubts.
Feel free to ask in the comments if you have any questions.
Also let me know, has this article helped you or not?
Suggested Important articles for you:
- Periodic table of elements (Detailed guide + HD image)
- Metals on the periodic table
- Nonmetals on the periodic table
- Metalloids on periodic table
- Nonmetals on the periodic table
- Halogens on periodic table
- Alkali metals on periodic table
- Alkaline earth metals on periodic table
- Transition metals on Periodic table
- Periodic trends in periodic table
