This is a SUPER easy guide on Germanium element.
In fact, the table mentioned below is the perfect information box (Which gives you every single detail about the Germanium element in Periodic table.)
So if you want to know anything about Germanium element, then this guide is for you.
Let’s finish this very quickly.
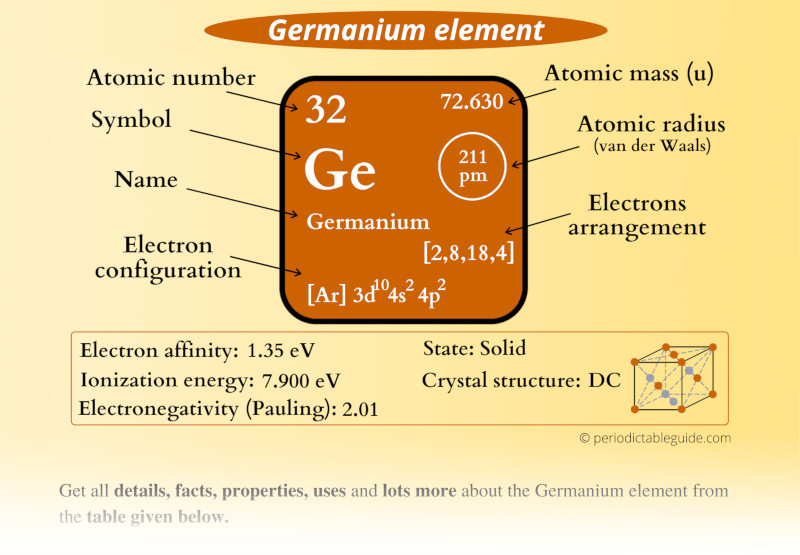
Germanium Element (Ge) Information
| Appearance |  Grayish white |
| State (at STP) | Solid |
| Position in Periodic table | 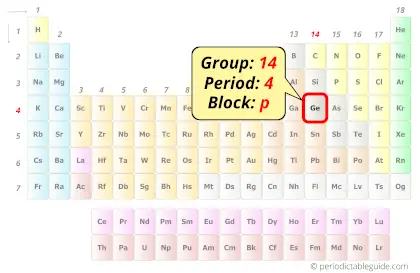 Group: 14, Period: 4, Block: p |
| Category | 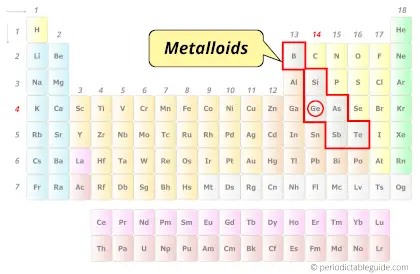 Metalloids |
| Atomic number or Protons | 32 |
| Neutrons | 41 |
| Electrons | 32 |
| Symbol | Ge |
| Atomic mass | 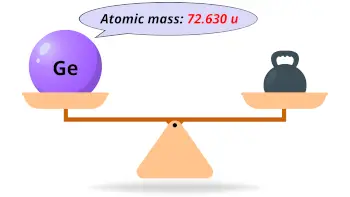 72.630 u |
| Electrons arrangement or Bohr model | 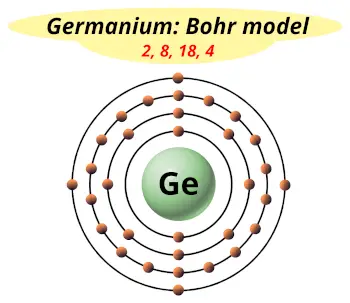 2, 8, 18, 4 |
| Electronic configuration | [Ar] 3d10 4s2 4p2 |
| Atomic radius | 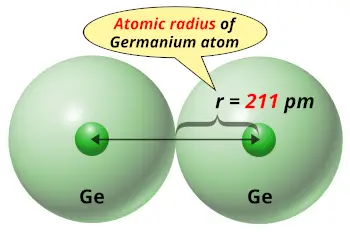 211 picometers (van der Waals radius) |
| Valence electrons | 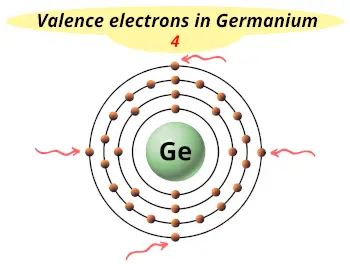 4 |
| 1st Ionization energy | 7.9 eV |
| Electronegativity | 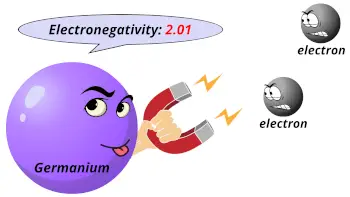 2.01 (Pauling scale) |
| Crystal structure | 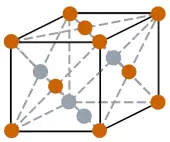 DC (Diamond cubic) |
| Melting point | 1211.40 K or 938.2 °C or 1720.8 °F |
| Boiling point | 3106 K or 2833 °C or 5131 °F |
| Density | 5.323 g/cm3 |
| Main isotope | 74Ge |
| Who discovered Germanium and when? |  Clemens Winkler in 1886 |
| CAS number | 7440-56-4 |
Germanium in Periodic table
Germanium element is in group 14 and period 4 of the Periodic table. Germanium is the p-block element and it belongs to the Metalloids – carbon group.
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba | La* | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| *Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| **Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
←Move to: Gallium (Ga) element – Periodic Table
→Move to: Arsenic (As) element – Periodic Table
What type of Element is Germanium? (Metal, Nonmetal or Metalloid?)
Germanium is a Metalloid (that means it shows properties of both metals as well as nonmetals.)
It shows few traits of metals as well as few traits of nonmetals.
In other words, Germanium shows properties that are a mixture of metallic properties as well as nonmetallic properties.
Germanium element has a metallic luster but it is not a metal.
It is neither a good conductor of electricity like metals nor a bad conductor of electricity like nonmetals.
It is neither hard like metals nor soft like nonmetals, but it is brittle in nature.
Hence, as germanium shows properties of both metals as well as non metals, it is classified as metalloid.
Also see:
1). Metals, Nonmetals and Metalloids on periodic table (Image)
2). How many Metalloids are on periodic table?
Why Germanium is called Semiconductor?
Germanium is called semiconductor because it is not a good conductor like metals and also not a bad conductor like nonmetals.
It has the conductivity which is higher than nonmetals, but lower than metals.
Hence, Germanium is called semiconductor.
Let me explain this with a simple example by using the concept of conduction band and valence band.
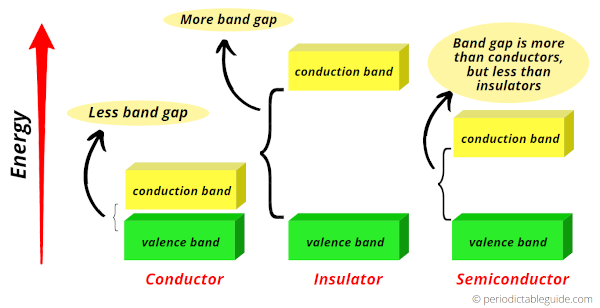
As shown in the above image, the green color band is the valence band while the yellow color band is the conduction band.
In conductors, the valence band and conduction band are very close to each other.
Hence, the free electrons can easily jump from valence band to conduction band.
Due to the more electron transfer from valence band to conduction band, these elements (for example: metals) show more conductivity.
Now in nonmetals, the gap between valence band and conduction band is very large.
So the free electrons do not transfer easily.
Hence in nonmetals, the conductivity is very less.
Now if we talk about germanium, then it is a Metalloid.
In Germanium, the gap between conduction band and valence band is more than metals and less than nonmetals.
So it is neither a good conductor nor a bad conductor of electricity.
Hence, Germanium is known as semiconductor.
Also see: Why is periodic table called periodic table?
Why is Germanium named Germanium?

In 1869, Mendeleev had predicted the existence of few unknown elements during his time.
He left gaps for the undiscovered element in his Periodic table.
In 1886, a new element was discovered with a high concentration of silver in it.
Clemens Winkler analyzed this new element and he found that it was a combination of silver, sulfur and one another unknown element.
He found that this new element had similar properties as that of antimony.
He decided to name this new element as “Neptunium”, because neptune planet was recently discovered during that time (in 1846).
But he found that there was already an existing element with the same name.
So he finally decide to give a name “Germanium” to this new element.
The word germanium came from the Latin word “Germania” for Germany.
He gave this name to honor his homeland Germany.
Why is Germanium used in camera lenses?

Germanium oxide (GeO2) is basically used in camera lenses, fibre optics, microscopes as well as infrared optics.
Germanium gives a special properties to the camera lenses.
Also, germanium oxide has higher index of refraction (because of higher index of refraction, the light travels slower through the lense).
Because of these reasons, wide angle camera lenses can be manufactured using germanium.
Germanium windows and lenses are also used for infrared thermography (as a front optic in thermal imaging cameras).
Does Germanium float in water?
No, germanium does not floats on water.
Let me explain this to you.
You might have studied the concept of density in physics.
By knowing the density of two objects, we can come to know which object will float and which will sink in water.
If the object has a density higher than the water, then it will sink. And if the density of object is less than the water, then it will float on water.
Now let’s see about germanium metal and it’s density.
Germanium has a density of 5.323 g/cm3, while water has a density of 1 g/cm3.
That means germanium metal is more dense than water.
Because of this reason, the Germanium metal sinks in water.
Also see: Does sodium floats on water?
Interesting Facts about Germanium
Interesting facts about germanium are mentioned below.
- Germanium is the element which expands on freezing, just like water does.
- The word germanium came from the Latin word “Germania” for Germany.
- Abundance of germanium in Earth’s crust is about 1.5 ppm (parts per million) by weight.
- In 1869, Mendeleev predicted the element “eka-silicon”, and later on that predicted element was named as Germanium.
- Researchers discovered the semiconducting properties of Germanium in 1945. Before this year, the use of Germanium element was very less.
- Out of the total germanium produced worldwide, around 85% of its consumption is in fibre optics.
- China is a leading producer of germanium.
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
External resources:
- The New Element, Germanium. (n.d.). Scientific American. https://doi.org/10.1038/scientificamerican11271886-9088bsupp
- Rochow, E. G. (1963, March). THE UNIQUE ELEMENT, GERMANIUM. Industrial & Engineering Chemistry, 55(3), 32–35. https://doi.org/10.1021/ie50639a007
- Germanium – Element information, properties and uses | Periodic Table. (n.d.). Germanium – Element Information, Properties and Uses | Periodic Table. https://www.rsc.org/periodic-table/element/32/germanium
- P. (n.d.). Germanium | Ge (Element) – PubChem. Germanium | Ge (Element) – PubChem. https://pubchem.ncbi.nlm.nih.gov/element/Germanium
- It’s Elemental – The Element Germanium. (n.d.). It’s Elemental – the Element Germanium. https://education.jlab.org/itselemental/ele032.html
