This is a SUPER easy guide on Krypton element.
In fact, the table mentioned below is the perfect information box (Which gives you every single detail about the Krypton element in Periodic table.)
So if you want to know anything about Krypton element, then this guide is for you.
Let’s finish this very quickly.
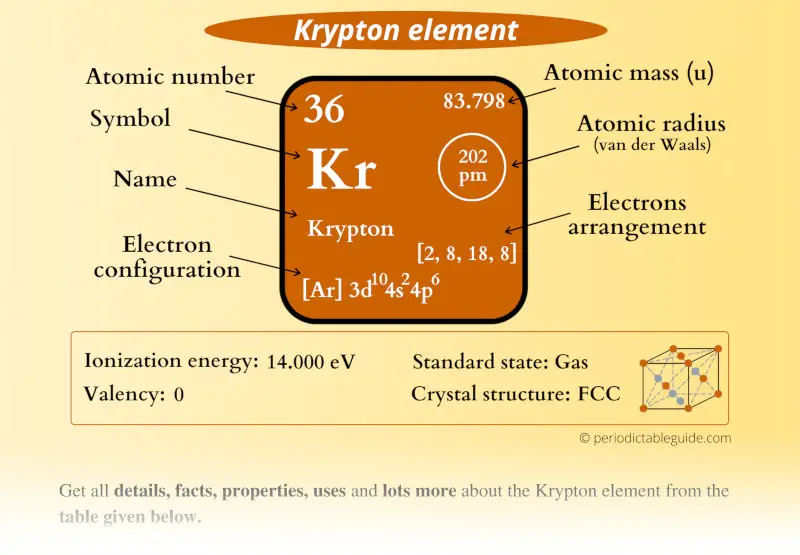
Krypton Element (Kr) Information
| Appearance | Colorless gas |
| State (at STP) | Gas |
| Position in Periodic table | 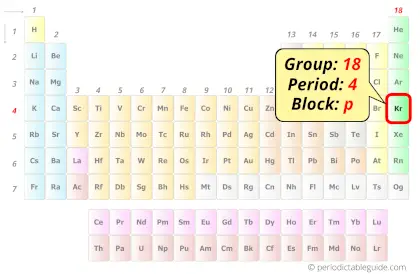 Group: 18, Period: 4, Block: p |
| Category | 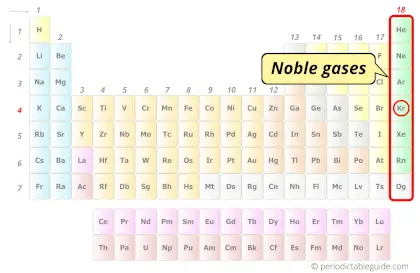 Noble gases |
| Atomic number or Protons | 36 |
| Neutrons | 48 |
| Electrons | 36 |
| Symbol | Kr |
| Atomic mass |  83.798 u |
| Electrons arrangement or Bohr model | 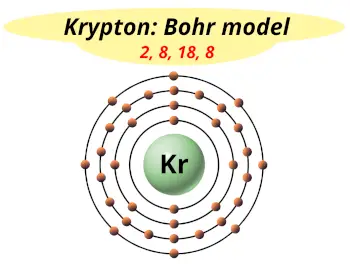 2, 8, 18, 8 |
| Electronic configuration | [Ar] 3d10 4s2 4p6 |
| Atomic radius | 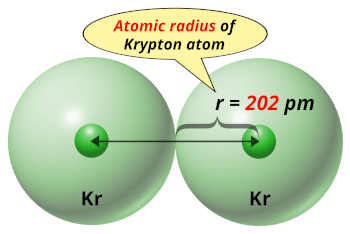 202 picometers (van der Waals radius) |
| Valence electrons | 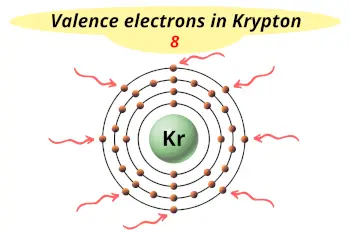 8 |
| 1st Ionization energy | 14.00 eV |
| Electronegativity | 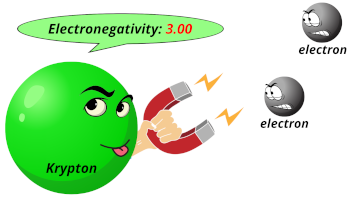 3.00 (Pauling scale) |
| Crystal structure | 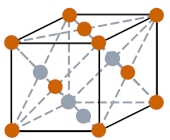 FCC (Face centered cubic) |
| Melting point | 115.7 K or -157.3 °C or -251.2 °F |
| Boiling point | 119.9 K or -153.4 °C or -244.1 °F |
| Density | 3.75 g/L |
| Main isotope | 84Kr |
| Who discovered Krypton and when? |  William Ramsay and Morris Travers (in 1898) |
| CAS number | 7439-90-9 |
Krypton in Periodic table
Krypton element is in group 18 and period 4 of the Periodic table. Krypton is the p-block element and it belongs to noble gases group.
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba | La* | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| *Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| **Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
←Move to: Bromine (Br) element – Periodic Table
→Move to: Rubidium (Rb) element – Periodic Table
Why is Krypton in Group 18?
Do you know, how many electrons can be accommodated in the first shell, second shell, third shell, fourth shell, etc…?
Here is the table showing the capacity of orbits to hold electrons.
Number of electrons in shells.
| Orbit / Shell (n) | Maximum no. of electrons this orbit can hold |
| K shell, n = 1 | 2 × 1² = 2 |
| L shell, n = 2 | 2 × 2² = 8 |
| M shell, n = 3 | 2 × 3² = 18 |
| N shell, n = 4 | 2 × 4² = 32 |
Thus,
- 1st shell can hold 2 electrons.
- 2nd shell can hold 8 electrons.
- 3rd shell can hold 18 electrons.
- 4th shell can hold 32 electrons.
Now the atomic number of krypton (Kr) is 36.
Hence the krypton element has electrons arrangement 2, 8, 18, 8.
This electron arrangement indicates that the outermost orbit of Krypton element (Kr) has 8 electrons.
Hence, it lies in group 18.
Why is Krypton in Period 4?
Let me ask you a question.
How many shells does krypton have?
It’s 4. Right?
You have already seen the bohr model of krypton atom in the above table.
From the Bohr model, it can be found that the number of orbits or shells in krypton is 4. Hence, as krypton has 4 orbits, it lies in period 4 of the Periodic table.
Why is Krypton in p-block?
Before knowing this reason, first of all I want to ask you a simple question.
How can you determine the blocks-wise position of elements?
The simple answer: The elements will lie in the s, p, d or f block will completely depend upon the subshell in which the last electron will enter.
For example; the electron configuration of krypton is [Ar] 3d10 4s2 4p6.
So the last electron of krypton enters the p-subshell or p-orbital.
Hence, krypton is the p-block element.
5 Interesting facts about Krypton
Interesting facts about krypton element are mentioned below.
- The name krypton is derived from the Greek word “kryptos” meaning hidden.
- Krypton is present in very less quantity in the earth’s atmosphere (around 1 ppm by volume).
- The atmosphere of Mars contains only 0.3 ppm (by volume) of krypton gas.
- When krypton gas is exposed to electric current under low pressure, it glows with a smoky white light.
- Krypton is one of the products that is obtained from the fission of uranium.
Properties of Krypton
The physical and chemical properties of krypton element are mentioned below.
Physical properties of Krypton
Physical properties of krypton are mentioned below.
- Krypton is colorless, odourless and tasteless gas.
- The atomic mass of krypton is 83.798 u and its density is 3.75 g/L.
- The melting point of krypton is -157.3 °C and its boiling point is -153.4 °C.
- Krypton has various stable isotopes as well as synthetic isotopes. Out of these isotopes, 84Kr is most abundant (around 57%).
- The van der Waals radius of the krypton atom is 202 picometers.
- The crystal structure of krypton element is FCC (face centered cubic).
Chemical properties of Krypton
Chemical properties of krypton are mentioned below.
- The electron shell configuration of krypton is 2, 8, 18, 8. This indicates that it has a completely filled outer shell, which makes it chemically stable.
- As krypton is chemically stable, it does not react with any other elements. And hence it does not have more chemical properties.
- It has been found that krypton reacts with fluorine to form krypton difluoride (KrF2) under certain conditions.
Uses of Krypton
Uses of krypton are mentioned below.
- Krypton is used in incandescent lights to protect the filament from oxygen.
- As krypton is an inert gas, it is also used in double glazed windows in the space between the two panes, which provides thermal insulation. (but in most cases, argon is preferred for this application as argon is cheaper than krypton.)
- 81Kr is used to determine the age of ice present in Antarctica.
- As krypton produces a bright white light, it is used for flashes in photography.
- One of the isotopes of krypton also finds its applications in MRI technology.
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
External resources:
- Krypton – American Chemical Society. (2018, December 3). American Chemical Society. https://www.acs.org/molecule-of-the-week/archive/k/krypton.html
- Krypton – Element information, properties and uses | Periodic Table. (n.d.). Krypton – Element Information, Properties and Uses | Periodic Table. https://www.rsc.org/periodic-table/element/36/krypton
- Krypton – Wikipedia. (2016, June 23). Krypton – Wikipedia. https://en.wikipedia.org/wiki/Krypton
- P. (n.d.). Krypton | Kr (Element) – PubChem. Krypton | Kr (Element) – PubChem. https://pubchem.ncbi.nlm.nih.gov/element/Krypton
- It’s Elemental – The Element Krypton. (n.d.). It’s Elemental – the Element Krypton. https://education.jlab.org/itselemental/ele036.html
