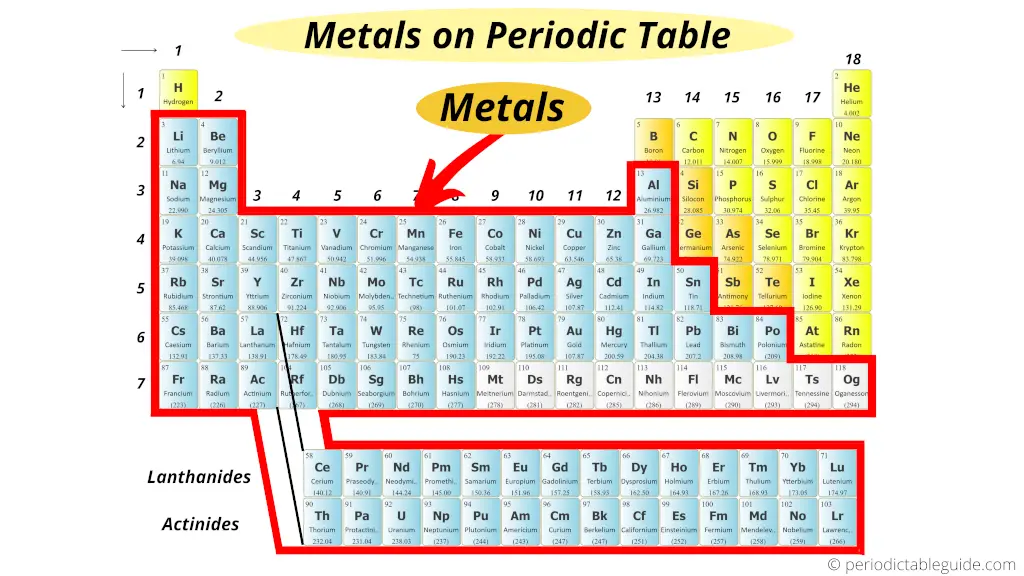
The metals are located on the left side of the Periodic Table.
This is the short and simple answer of your question “Where are Metals located on the Periodic Table?”
But wait…
There are lot more things you need to know about the metals of the Periodic table, like;
- Alkali metals on Periodic table
- Alkaline Earth metals on Periodic table
- Transition metals on Periodic table
- Inner transition metals on Periodic table
- How many metals are there on Periodic table?
- Rare Earth metals on Periodic table
- Heavy metals on Periodic table
- Reactive metals on Periodic table
- The complete list of all metals
- Why are metals on the left of Periodic table?
- Properties of metals
- And lots more…
Let’s dive straight into it.
What are metals in Periodic table?
Metals are the elements which have the tendency to donate or lose electrons to form positive ions.
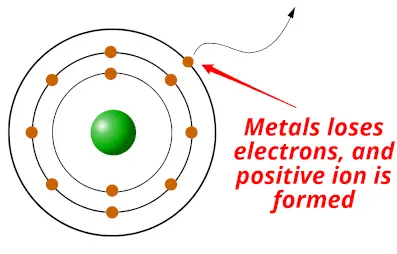
In short remember that;
- Metals are electron donors (metals loses / donates electrons)
The elements which show this type of nature (nature of losing electrons) are known as metals.
Now in the Periodic table, these metals are found on the left side.
But these metals are further classified into many more types.
Let me tell you few things about the types of metals on the Periodic table.
Alkali Metals on Periodic table
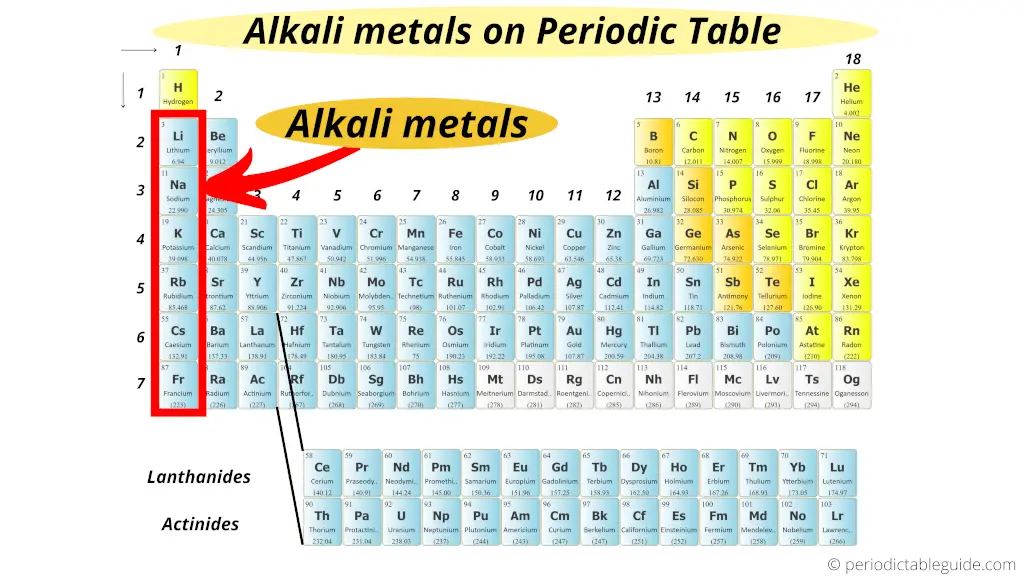
The left most elements on the Periodic table (group 1 elements) are known as alkali metals.
But do you know;
What are alkali metals? And why the alkali metals are called so?
The simple answer: Alkali metals form an alkaline solution (basic solution) when they react with water.
That’s why they are known as alkali metals. (Read detailed explanation from here.)
Also visit: Why are alkali metals so reactive?
Alkali Earth Metals on Periodic table
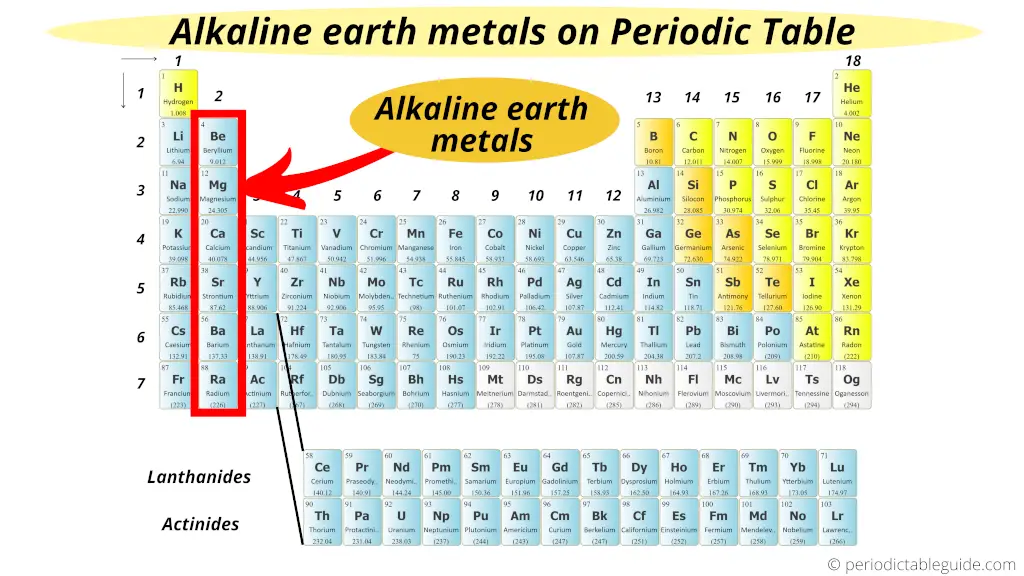
The Alkaline Earth metals are located in the 2nd group of Periodic table.
Now again I will tell you a simple reason why these group 2 elements are known as Alkaline Earth Metals.
See, the group 2 elements have almost similar properties that of the group 1 elements (Alkali Metals).
But the difference is that they are mostly found from the earth crust.
Thus they are known as Alkaline Earth Metals.
Transition Metals on Periodic table
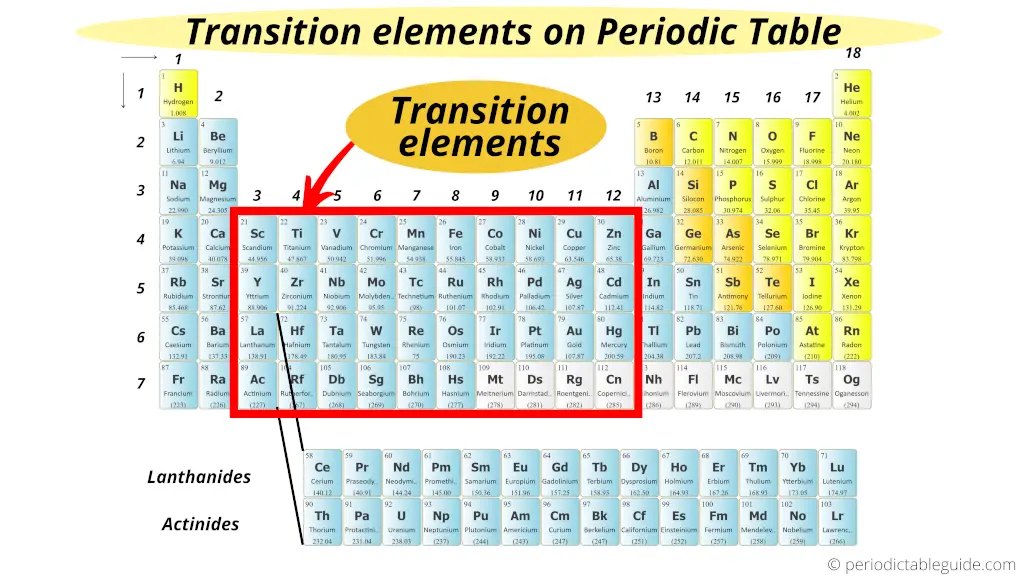
The elements lying in group 3 to group 12 are known as Transition metals (or transition elements).
Transition metals form a bridge between the chemically active metals of s-block elements and the less active elements of Groups 13 and 14.
Thus these metals are known as “Transition metals”.
Also visit: How many transition metals are there on periodic table?
Inner Transition Metals on Periodic table
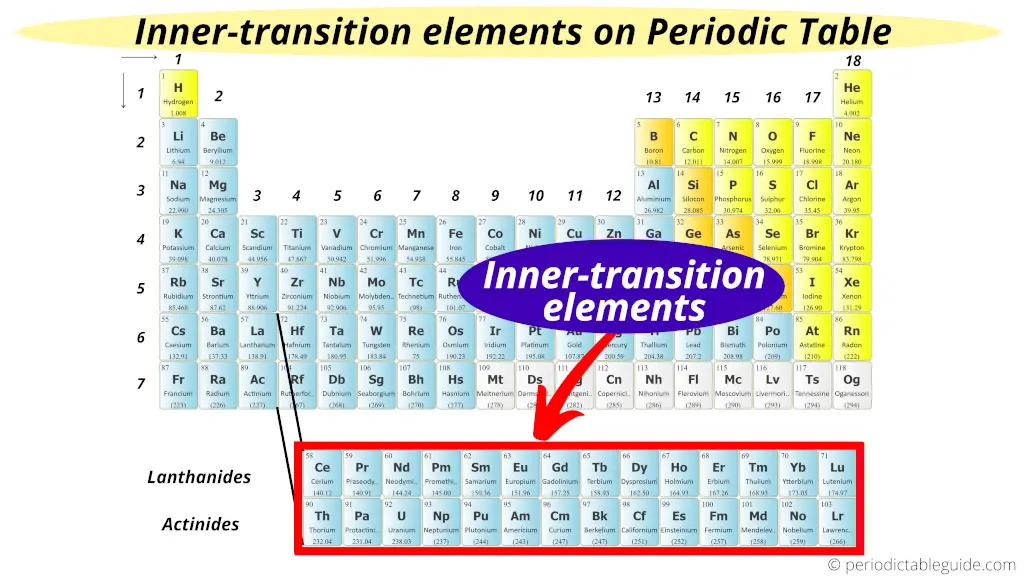
The two bottom rows in the Periodic table are called inner transition metals.
Why?
Just as the name suggests, they are transition metals only, but they are lying in the inner section of the transition metals.
Hence they are named as inner transition metals (or inner transition elements)
The elements of the first row are known as Lanthanides and the elements of the second row are known as Actinides. [1]
Also visit:
1). Why are inner transition metals at the bottom of periodic table?
2). Difference between transition and inner transition elements.
How many metals are there on Periodic table?
Short answer: I don’t know exactly (lol…)
Let me explain to you why I do not know this answer.
A general question which all students have is about the total number of metals on the Periodic table.
Well, there is not the exact answer to this question.
But out of the total 118 known elements, more than 78% of the elements show metallic character.
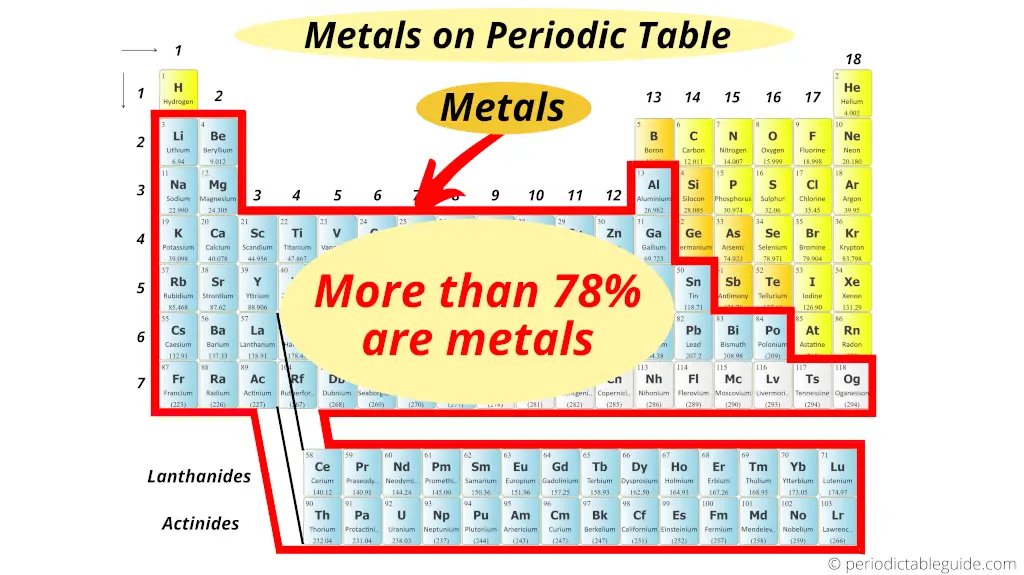
Out of the 118 elements of periodic table, approximately 94 elements are metals (but this is not the exact number).
Reason?
According to wikipedia, this number is inexact because the boundaries between metals, nonmetals and metalloids fluctuate slightly due to lack of universally accepted definitions.
(That means in metallurgy, the researchers may define metals on the basis of density. In physics, they may define metals on the basis of physical properties. And in chemistry, the chemists are concerned with the chemical properties of metals.)
Also the elements which are shown in grey color are still under research work by the researchers (elements 109 to 118 mentioned in grey color).
These are the synthetic elements and they have a very short half life.
This is the reason why the total number of metals are still not known.
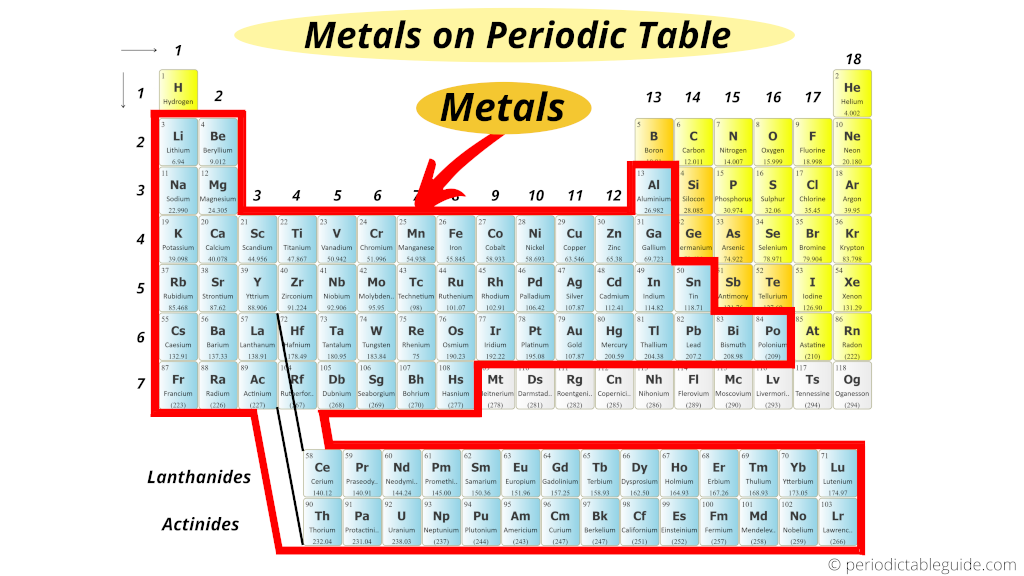
So to be exact, if we consider the first 108 elements, then there are total 84 metals present on Periodic table.
Rare Earth metals on Periodic table
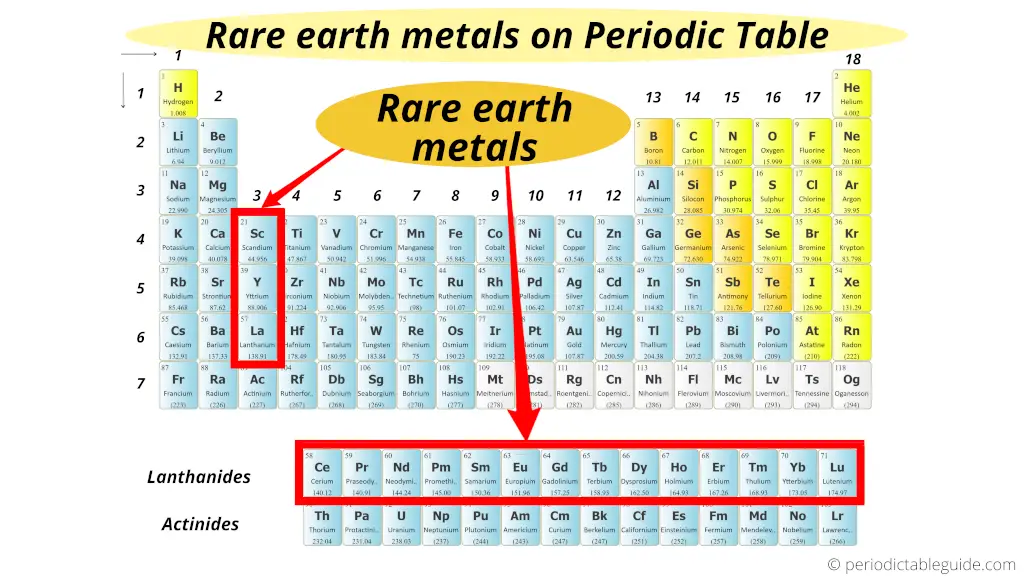
There are total 17 Rare Earth metals on the Periodic table. Rare Earth Metals includes all the 15 Lanthanides as well as scandium(Sc) and yttrium (Y). So total 15 + 2 = 17 Rare Earth metals.
Here is a complete list of all the 17 rare earth metals.
| Atomic number | Symbol | Name of element |
| 21 | Sc | Scandium |
| 39 | Y | Yttrium |
| 57 | La | Lanthanum |
| 58 | Ce | Cerium |
| 59 | Pr | Praseodymium |
| 60 | Nd | Neodymium |
| 61 | Pm | Promethium |
| 62 | Sm | Samarium |
| 63 | Eu | Europium |
| 64 | Gd | Gadolinium |
| 65 | Tb | Terbium |
| 66 | Dy | Dysprosium |
| 67 | Ho | Holmium |
| 68 | Er | Erbium |
| 69 | Tm | Thulium |
| 70 | Yb | Ytterbium |
| 71 | Lu | Lutetium |
But do you know why they are called rare?
Why Rare Earth metals are called so?
Answer: You might be thinking that rare means these elements are available in very less quantity.
But if you are thinking so, then you are wrong.
Rare earth metals are not actually that rare as the name suggests.
Even, they are available in more abundant quantities than gold.
But the fact is that they are spread evenly on the earth and it is very difficult to find these elements at one place on the earth.
Thus they are rare in the context of available resources.
Heavy metals on Periodic table
First of all,
What are heavy metals?
The heavy metals are those metals which possess higher density or higher atomic mass.
Well, the definition of heavy metals may differ in metallurgy, physics as well as chemistry.
In metallurgy, researchers define heavy metals on the basis of density.
While in physics, they may use atomic numbers for defining heavy metals.
Whereas in chemistry, chemists are concerned with the chemical properties of heavy metals for defining them.
So there is a research work still going on for the specific definition and classification of heavy metals.
Many researchers use the common criteria that if the metals have the density of more than 5 g/cm³, then they are likely to be known as heavy metals.
On the basis of this criteria, the heavy metals are shown on the Periodic table.
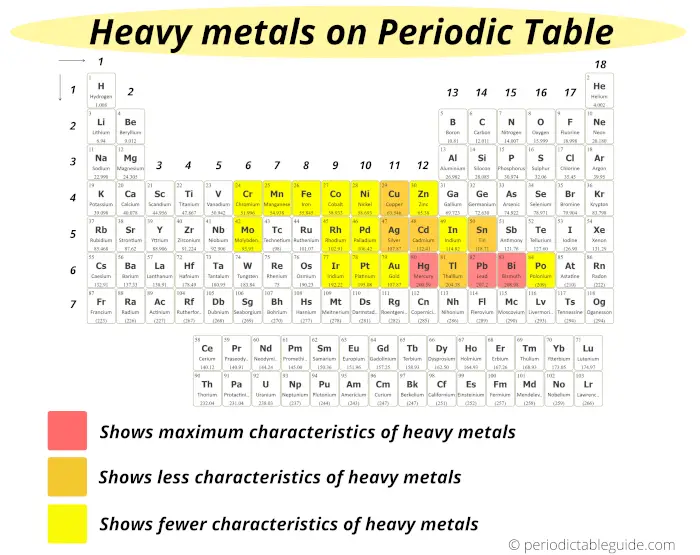
The metals which show maximum characteristics of heavy metals are;
These heavy metals are displayed on the Periodic table with red color (see above image)
The metals which show less characteristics of heavy metals are;
And these elements are represented by orange color on the Periodic table.
Rest of the elements which are shown in yellow color show fewer characteristics of heavy metals.
Reactive metals on Periodic table
Here I’ll tell you about the most reactive metal on the Periodic table. But before that, let us discuss what is a reactivity in metals?
What are reactive metals?
Reactive metals are those metals which show high tendency to lose electron/s in a chemical reaction.
For example, as we move down the group, the atomic size increases.
Thus the force of attraction between nucleus and outermost electrons decreases.
Because of this, the electron will be lost easily during chemical reaction.
Hence, down the group, as the atomic size increases, the reactivity of metals increases.
Most reactive metal on Periodic table
The highly reactive metals are located on the left bottom corner of the Periodic table. They are the Alkali metals of group 1.
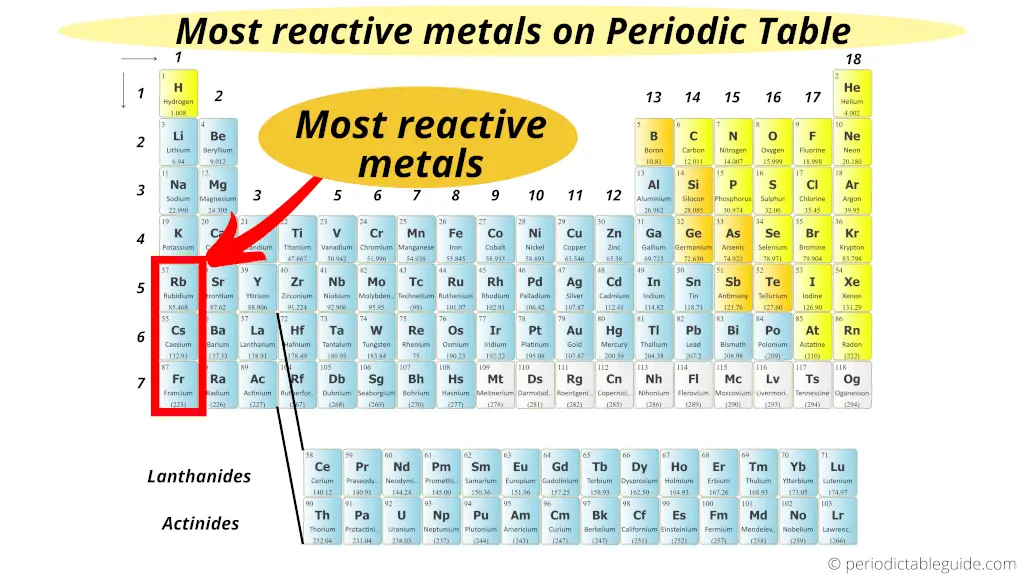
In 1st group, as we move down from top to bottom, the reactive of metals increases.
Thus the bottom most element of group 1 (i.e francium) is the most reactive metal on the Periodic table.
(Note: Francium is a laboratory made element. It is available in a very less quantity. And hence for any practical purposes, caesium is considered as the most reactive metal in Periodic table. Caesium has high reactivity, but it is predicted that Francium would have even higher reactivity than that of caesium)
Also see: Reactivity of alkali metals with water (Explained with animation)
List of all the metals on the Periodic table
Why are metals on the left of Periodic table?
Do you know what is the main property of metal?
They lose electron/s during the chemical reaction.

Now just think, what should be the conditions or properties that an element should possess to make the electron lost easily.
Here are few properties necessary for easy loss of electrons.
- Element should have low ionization energy
- Element should have less number of electrons in outermost orbit
- Element should have less electronegativity
If the elements possess these properties, then they will behave like metals.
Now, you know that the elements on the left side of Periodic table have less valence electrons in their outermost orbit.
Also the atomic size of these elements are more, which is favorable for the loss of electrons.
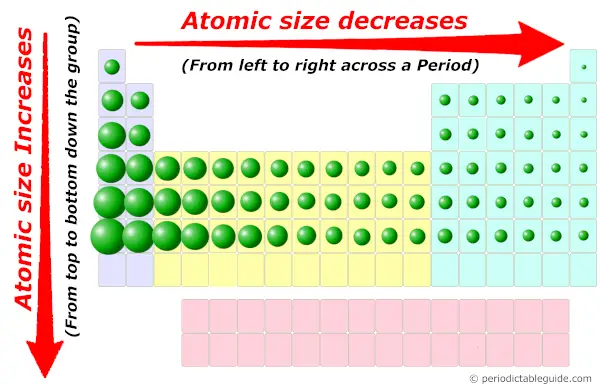
As they possess bigger atomic size, it is easy to remove the valence electrons from the outermost orbit. So they have low ionization energy.
Also these elements located on the left side have less electronegativity.
Let me give you a short introduction about electronegativity.
Electronegativity is a tendency to attract the electron pair.
I have discussed this in detailed article of Periodic table that electronegativity depends upon the size of an atom.
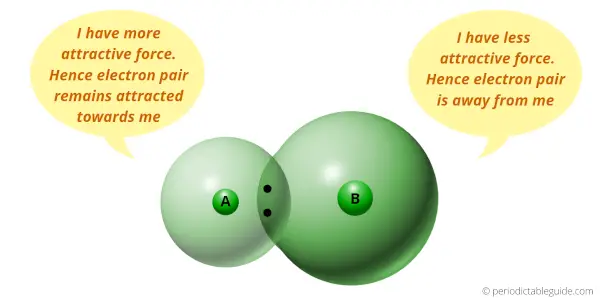
If atomic size is less, then it has more tendency to attract the electron pair (means smaller the size, more is the electronegativity.) And if the atomic size is more, then it has less tendency to attract the electron pair (means bigger the size, lesser is the electronegativity.)
Now the elements on the left side of Periodic table have more atomic size. So they will have less electronegativity.
Hence, they are more metallic in nature.
I hope, now you have got the answer of “Why metals are located on the left side of Periodic table?”
Properties of metals
Here I’ll show you the physical and chemical properties of metals.
Keep reading…
Physical properties of metal
#1 Sonorous

Metals produce ringing sound when they are stuck hard. This indicates that metals are sonorous in nature.
For example: Ringing of bell.
#2 Solid state at room temperature
All the metals are solids at room temperature (except mercury)
They have definite shape and size.
#3 Lustrous (shiny)

Metals are Lustrous.
They have a smooth shiny surface.
#4 Malleability

Metals can be reshaped into thin sheets on applying sufficient pressure on it. This property of metals is known as malleability.
#5 Ductility
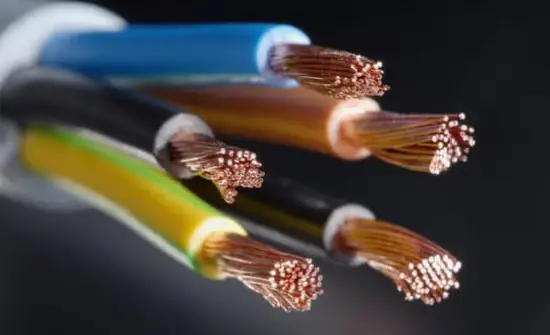
Metals can be drawn into thin wires. (For example cable wires, thin copper wires used in electric circuit used in your laptop or phone which you are holding right now, etc …)
#6 Conductivity

Metals are good conductor of heat as well as electricity.
That means heat and electricity can easily pass through the metals.
#7 High melting point and boiling point

The metals have a high melting point as well as boiling point.
#8 Hardness

Hardness is the ability of a material to resist wear, tear, scratching and to resist the changes in shape.
Generally, all the metals show these properties (except mercury)
(Note: Sodium, potassium and lithium are soft metals which can be cut with a kitchen knife.)
#9 Density

Metals have high density. And because of this, metals have more weight.
Chemical properties of metal
#1 Valency
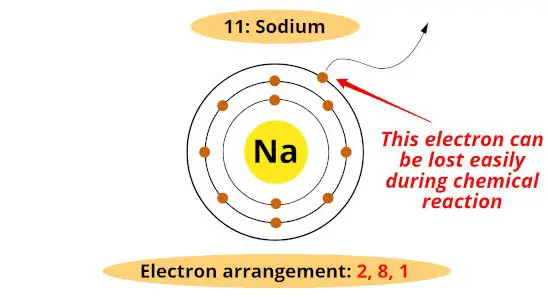
The atoms or metals have generally 1, 2 or 3 electrons in the outermost orbit, and they lose these electrons during a chemical reaction.
#2 Corrosiveness

Some metals get corroded easily when they are exposed to moist air or water (For example: Iron)
#3 Loss of electrons

Metals have a tendency to lose electrons. They donate electrons during a chemical reaction.
#4 Low electronegative
Metals are less electronegative and so they are electropositive in nature.
#5 Reactive
Some metals are chemically more reactive. They undergo chemical reactions by themselves or other elements and release energy.
For example: Potassium gets ignited automatically when it is exposed to water.
#6 Forms basic oxides
Metals react with oxygen and form basic oxides.
#7 Good reducing agents
Metals are reducing agents because they donate electron/s during a chemical reaction and get oxidized.
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
Summary
So in the entire article, we have discussed the location of Metals on the Periodic table.
We saw the;
- Position of Alkali Metals,
- Position of Alkaline Earth Metals,
- Position of transition metals,
- Position of Inner transition metals,
- Position of Rare earth metals,
- Position of Heavy metals as well as reactive metals.
Then we discussed the complete list of all the metals of the Periodic table.
I gave you the reason why the metals are located on the left side of the Periodic table.
And finally we discussed the physical and chemical properties of metals.
I hope this article “Where are Metals located on the Periodic Table” has helped you in solving your queries.
Suggested Important articles for you:
- Everything you need to know about the periodic table
- Periodic table with different types of metals
- Nonmetals on the periodic table
- Metalloids on the periodic table
- Periodic table labeled with metals nonmetals and metalloids
- Halogens on Periodic table
- Noble gases on periodic table
- List of elements of Periodic table
- Periodic table with electron configuration
- How are the elements arranged in the modern periodic table?
