This is a SUPER easy guide on Rhodium element.
In fact, the table mentioned below is the perfect information box (Which gives you every single detail about the Rhodium element in Periodic table.)
So if you want to know anything about Rhodium element, then this guide is for you.
Let’s finish this very quickly.
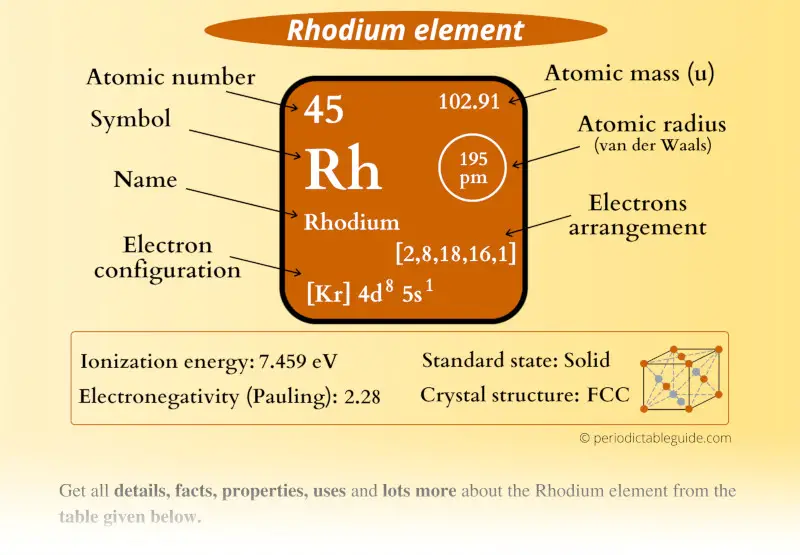
Rhodium Element (Rh) Information
| Appearance |  Silvery white metallic luster |
| State (at STP) | Solid |
| Position in Periodic table | 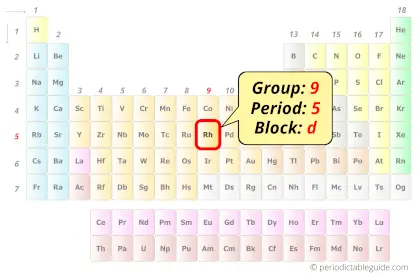 Group: 9, Period: 5, Block: d |
| Category | 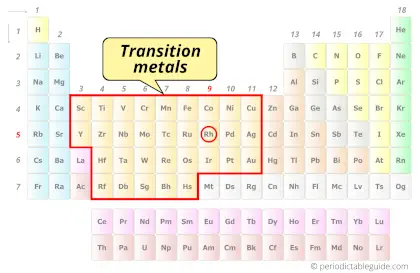 Transition metals |
| Atomic number or Protons | 45 |
| Neutrons | 58 |
| Electrons | 45 |
| Symbol | Rh |
| Atomic mass | 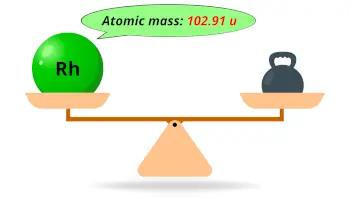 102.91 u |
| Electrons arrangement or Bohr model | 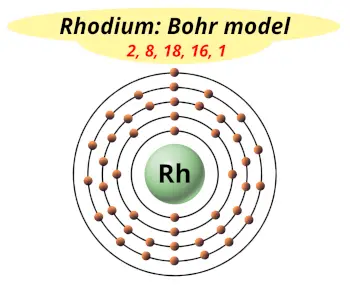 2, 8, 18, 16, 1 |
| Electronic configuration | [Kr] 4d8 5s1 |
| Atomic radius | 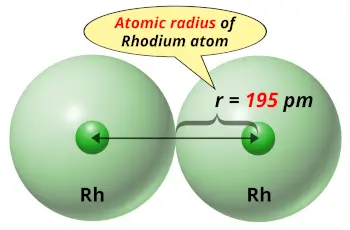 195 picometers (van der Waals radius) |
| 1st Ionization energy | 7.459 eV |
| Electronegativity | 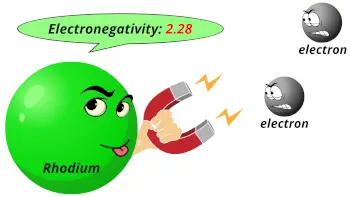 2.28 (Pauling scale) |
| Crystal structure | 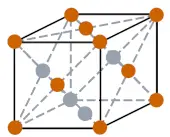 FCC (Face centered cubic) |
| Melting point | 2237 K or 1964 °C or 3567 °F |
| Boiling point | 3968 K or 3695 °C or 6683 °F |
| Density | 12.45 g/cm3 |
| Main isotope | 103Rh |
| Who discovered Rhodium and when? |  William Hyde Wollaston in 1804 |
| CAS number | 7440-16-6 |
Rhodium in Periodic table
Rhodium element is in group 9 and period 5 of the Periodic table. Rhodium is the d-block element and it belongs to transition metals group.
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba | La* | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| *Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| **Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
←Move to: Ruthenium (Ru) element – Periodic Table
→Move to: Palladium (Pd) element – Periodic Table
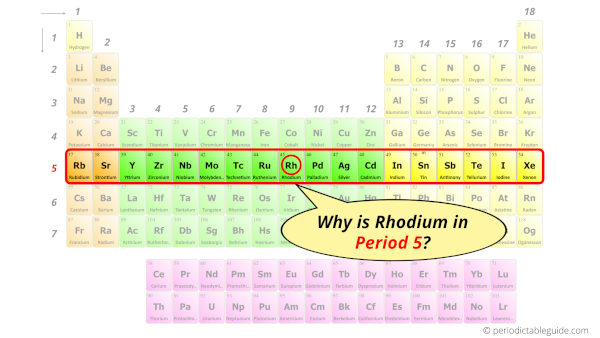
Why is Rhodium in Period 5?
Let me ask you a question.
How many shells does Rhodium have?
It’s 5. Right?
You have already seen the bohr model of rhodium atom in the above table.
From the Bohr model, it can be found that the number of orbits or shells in rhodium is 5. Hence, as rhodium has 5 orbits, it lies in period 5 of the Periodic table.
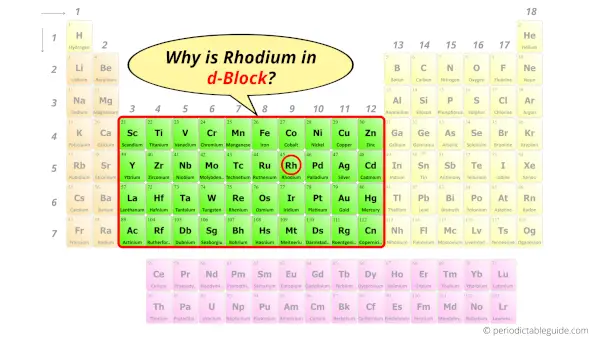
Why is Rhodium in d-block?
Before knowing this reason, first of all I want to ask you a simple question.
How can you determine the blocks-wise position of elements?
The simple answer: The elements will lie in the s, p, d or f block will completely depend upon the subshell in which the last electron will enter.
For example; the electron configuration of rhodium is [Kr] 5s1 4d8.
So the last electron of rhodium enters the d-subshell or d-orbital.
Hence, rhodium is the d-block element.
Is Rhodium a Transition Metal? Why?
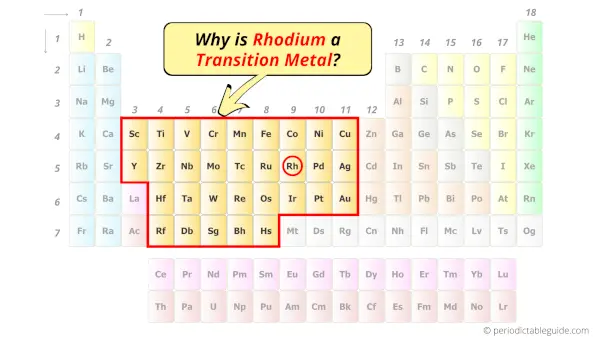
Yes, Rhodium is a transition metal because it has incompletely filled d-orbital in its ground state.
Let me explain the exact meaning of this.
According to the definition of transition metals;
The element should compulsorily have incomplete d-orbitals, either in their ground state (M) or most common oxidation states (M1+, M2+, etc) then only they are called transition metals.
Now, the ground state of Rhodium means its normal state in which it has neither gained nor lost any electron/s.
So the ground state of Rhodium is Rh.
And the ground state electronic configuration of Rhodium is [Kr] 5s1 4d8.
In this state, if we see the electron configuration of Rhodium, then it possesses incomplete d-orbitals.
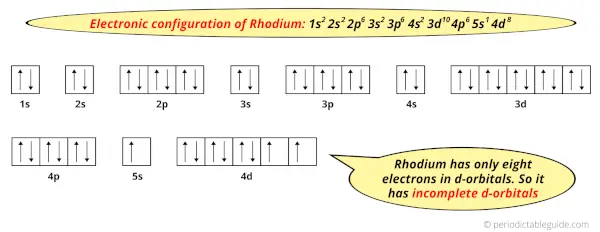
Because, there are only eight electrons in the d-orbitals.
In order to have the complete d-orbitals, there must be 10 electrons in it.
But in the ground state electronic configuration of rhodium, you can see that it has only 8 electrons in d-orbitals.
Thus, Rhodium has incomplete d-orbitals.
And hence, as Rhodium has incomplete d-orbitals, it is considered as a transition metal.
5 Interesting facts about Rhodium
Interesting facts about rhodium element are mentioned below.
- The name rhodium was derived from the Greek word “Rhodon” which means rose.
- Rhodium is a very rare metal and its proportion in earth’s crust is only 1 part per 200 million. [1]
- Rhodium was discovered by William Hyde Wollaston in 1804. He was also the person behind the discovery of palladium.
- Out of the total available rhodium, around 87% of rhodium is used in catalytic converters.
- Rhodium is a very rare metal and it can be obtained from the waste product of uranium fission.
Properties of Rhodium
The physical and chemical properties of rhodium element are mentioned below.
Physical properties of Rhodium
Physical properties of rhodium are mentioned below.
- Rhodium is a solid metal having silvery white metallic luster.
- Rhodium is a hard reflective metal and it is resistant to wear.
- The melting point of rhodium is 1964 °C and its boiling point is 3695 °C.
- The atomic mass of rhodium is 102.91 u and its density is 12.45 g/cm3.
- The crystal structure of rhodium is FCC (Face centered cubic).
- Rhodium has a stable isotope (103Ru) which has an abundance of almost 100%. Besides this, there are many other radioactive isotopes of rhodium.
Chemical properties of Rhodium
Chemical properties of rhodium are mentioned below.
- Rhodium is a transition metal and it has incomplete d-subshells.
- Rhodium is a corrosive resistant metal.
- Rhodium does not react with oxygen easily and hence it is also known as a noble metal.
- Rhodium does not show any chemical reaction with air and water upto 600 °C temperature.
- Rhodium is also chemically very less reactive to most of the acids.
Uses of Rhodium
Uses of rhodium are mentioned below.
- Rhodium is an element which is used in catalytic converters that are used in cleaning the vehicle emissions.
- Rhodium is also used as a catalyst in chemical industries to prepare nitric acid as well as acetic acid.
- Rhodium is also used in coating of optical fibers as well as coating of crucibles.
- Rhodium is basically used as an alloying metal with platinum and iridium which provides resistance against higher temperatures.
Free Gift for you: Interactive Periodic Table
Let me tell you how this Interactive Periodic Table will help you in your studies.
1). You can effortlessly find every single detail about the elements from this single Interactive Periodic table.
2). You will get the detailed information about the periodic table which will convert a newbie into pro.
3). You will also get the HD images of the Periodic table (for FREE).
Checkout Interactive Periodic table and download it’s high resolution image now (It’s FREE)
External resources:
- Rhodium – Element information, properties and uses | Periodic Table. (n.d.). Rhodium – Element Information, Properties and Uses | Periodic Table. https://www.rsc.org/periodic-table/element/45/rhodium
- Rhodium – Wikipedia. (2007, December 10). Rhodium – Wikipedia. https://en.wikipedia.org/wiki/Rhodium
- P. (n.d.). Rhodium | Rh (Element) – PubChem. Rhodium | Rh (Element) – PubChem. https://pubchem.ncbi.nlm.nih.gov/element/Rhodium
- It’s Elemental – The Element Rhodium. (n.d.). It’s Elemental – the Element Rhodium. https://education.jlab.org/itselemental/ele045.html
- Rhodium (Rh) | AMERICAN ELEMENTS ® (n.d.). American Elements: The Materials Science Company. https://www.americanelements.com/rh.html