This is a SUPER easy guide on Palladium element.
In fact, the table mentioned below is the perfect information box (Which gives you every single detail about the Palladium element in Periodic table.)
So if you want to know anything about Palladium element, then this guide is for you.
Let’s finish this very quickly.
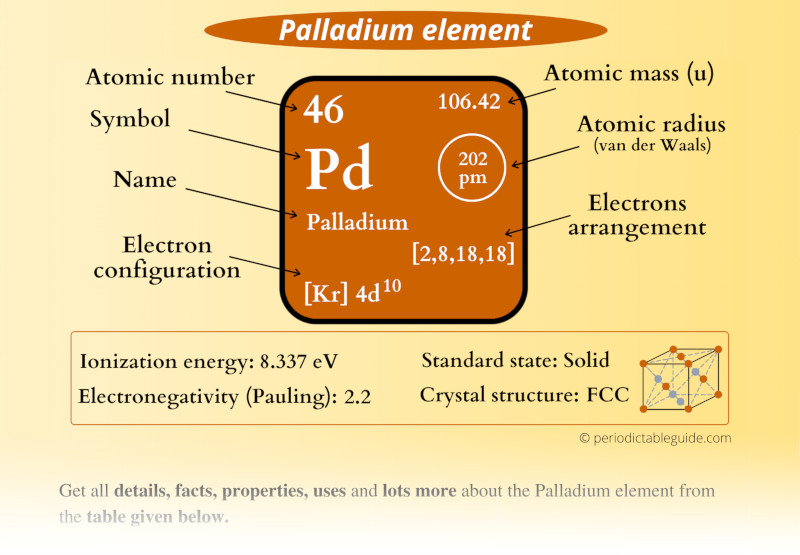
Palladium Element (Pd) Information
| Appearance |  Silvery white |
| State (at STP) | Solid |
| Position in Periodic table | 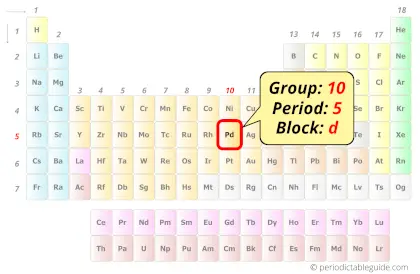 Group: 10, Period: 5, Block: d |
| Category | 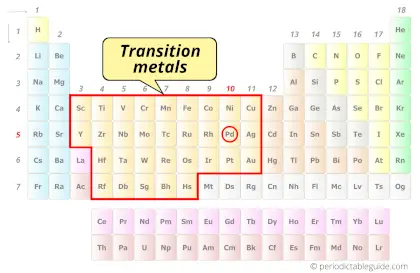 Transition metals |
| Atomic number or Protons | 46 |
| Neutrons | 60 |
| Electrons | 46 |
| Symbol | Pd |
| Atomic mass |  106.42 u |
| Electrons arrangement or Bohr model | 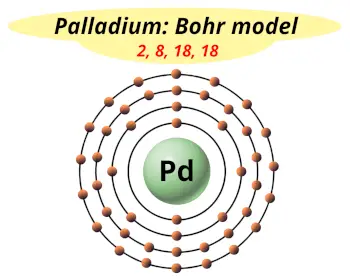 2, 8, 18, 18 |
| Electronic configuration | [Kr] 4d10 |
| Atomic radius | 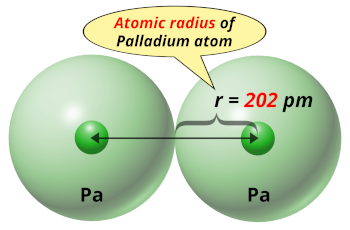 202 picometers (van der Waals radius) |
| 1st Ionization energy | 8.337 eV |
| Electronegativity | 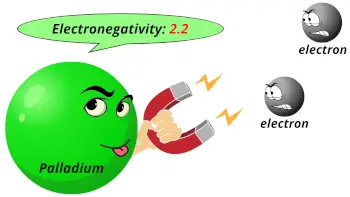 2.2 (Pauling scale) |
| Crystal structure | 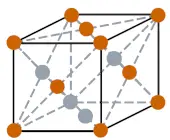 FCC (Face centered cubic) |
| Melting point | 1828 K or 1554.9 °C or 2830.8 °F |
| Boiling point | 3236 K or 2963 °C or 5365 °F |
| Density | 12.023 g/cm3 |
| Main isotope | 105Pd (22%), 106Pd (27%), 108Pd (26%) |
| Who discovered Palladium and when? |  William Hyde Wollaston in 1802 |
| CAS number | 7440-05-3 |
Palladium in Periodic table
Palladium element is in group 10 and period 5 of the Periodic table. Palladium is the d-block element and it belongs to transition metals group.
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba | La* | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| *Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| **Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
←Move to: Rhodium (Rh) element – Periodic Table
→Move to: Silver (Ag) element – Periodic Table
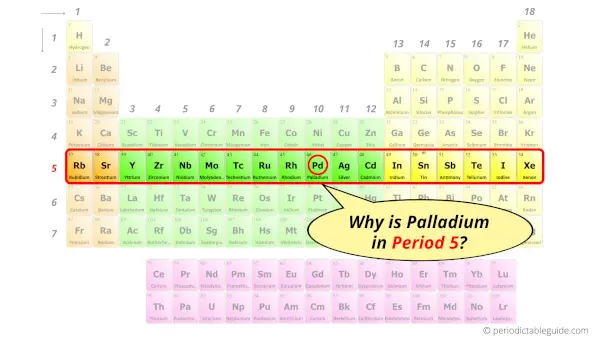
Why is Palladium in Period 5?
Let me ask you a question.
How many shells does palladium atom have?
It’s 5. Right?
You have already seen the bohr model of palladium atom in the above table.
From the Bohr model, it can be found that the number of orbits or shells in palladium is 5. Hence, as palladium has 5 orbits, it lies in period 5 of the Periodic table.
Why is Palladium in d-block?
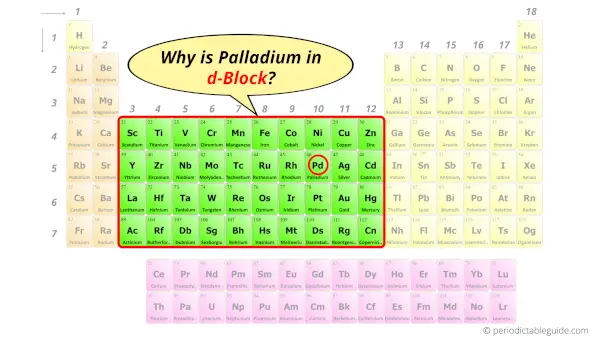
Before knowing this reason, first of all I want to ask you a simple question.
How can you determine the blocks-wise position of elements?
The simple answer: The elements will lie in the s, p, d or f block will completely depend upon the subshell in which the last electron will enter.
For example; the electron configuration of palladium is [Kr] 4d10.
So the last electron of palladium enters the d-subshell or d-orbital.
Hence, palladium is the d-block element.
Is Palladium a Transition Metal? Why?
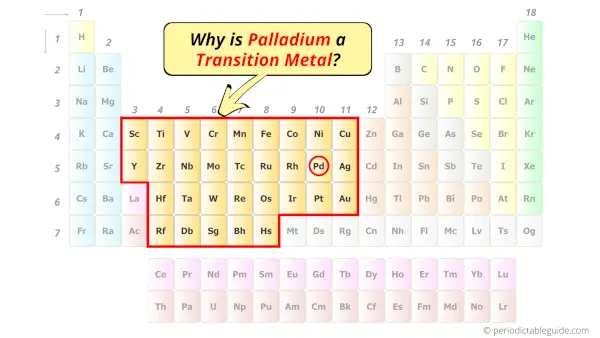
Yes, Palladium is a transition metal because it has incompletely filled d-orbital in its most common oxidation state (Pd2+).
Let me explain the exact meaning of this.
According to the definition of transition metals;
The element should compulsorily have incomplete d-orbitals, either in their ground state (M) or most common oxidation states (M1+, M2+, etc) then only they are called transition metals.
Now, the ground state of palladium means its normal state in which it has neither gained nor lost any electron/s (i.e Pd).
And the most common oxidation state of palladium is Pd2+, because most commonly palladium loses 2 electrons during a chemical reaction.
Now,
The electron configuration of Pd is: [Kr] 4d10 and
The electron configuration of Pd2+ is: [Kr] 4d8
So, in this most common oxidation state of palladium (Pd2+), if we see the electron configuration, then it possesses incomplete d-orbitals.
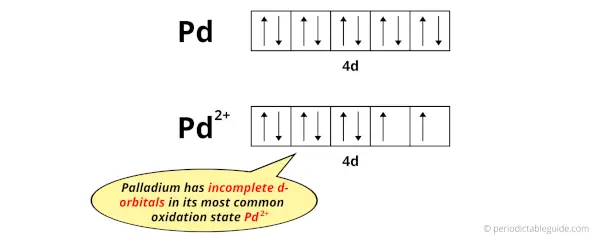
You can see that all the d-orbitals are completely filled (all the 10 electrons are present) in the elemental state (Pd) but they are incomplete (only 8 electrons are present) in their oxidation state (Pd2+).
In short, palladium element have incomplete d-orbitals in its oxidation state (Pd2+).
Hence, according to the definition mentioned above, palladium is a transition metal.
7 Interesting facts about Palladium
Interesting facts about palladium element are mentioned below.
- The name palladium came from the name of asteroid “pallas”.
- Palladium is rarer than precious metals like gold and platinum.
- Palladium is naturally found as an alloyed metal with gold and other metals of platinum group.
- Russia and South Africa are leading producers of palladium and these regions supply around 40% of annual production of palladium.
- Palladium was discovered by the chemist William Hyde Wollaston in 1802. He is also the person behind the discovery of rhodium element.
- Palladium has a capacity to absorb hydrogen upto 900 times of its volume. [1]
- More than 50% of palladium produced annually is used in catalytic converters.
Properties of Palladium
The physical and chemical properties of palladium element are mentioned below.
Physical properties of Palladium
Physical properties of palladium are mentioned below.
- Palladium is a metal having a silvery white appearance.
- The atomic mass of palladium is 106.42 u and its density is 12.023 g/cm3.
- The melting point of palladium is 1554.9 °C and its boiling point is 2963 °C.
- There are many isotopes of palladium, and out of them the most abundant stable isotope is 106Pd (around 27%).
- The crystal structure of palladium is FCC (Face centered cubic).
Chemical properties of Palladium
Chemical properties of palladium are mentioned below.
- Palladium is a transition metal because it possesses incomplete d-orbitals in its most common oxidation state Pd2+.
- Palladium does not tarnish when kept open in the air.
- Palladium reacts with oxygen at higher temperatures and forms palladium oxide.
- When palladium absorbs hydrogen, it forms palladium hydride.
Uses of Palladium
Uses of palladium are mentioned below.
- In chemical industries, Palladium is mainly used as a catalyst which speeds up the chemical reaction.
- As palladium does not tarnish in the air, it is also used in jewelry.
- Palladium is used as a catalytic converter in automobiles.
- As palladium has a capacity to absorb hydrogen, it is used in storing or filtering hydrogen.
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
External resources:
- Atomic Data for Palladium (Pd). (n.d.). Atomic Data for Palladium (Pd). https://physics.nist.gov/PhysRefData/Handbook/Tables/palladiumtable1.htm
- Palladium – Element information, properties and uses | Periodic Table. (n.d.). Palladium – Element Information, Properties and Uses | Periodic Table. https://www.rsc.org/periodic-table/element/46/palladium
- Palladium – Wikipedia. (2009, November 12). Palladium – Wikipedia. https://en.wikipedia.org/wiki/Palladium
- It’s Elemental – The Element Palladium. (n.d.). It’s Elemental – the Element Palladium. https://education.jlab.org/itselemental/ele046.html
- C&EN: IT’S ELEMENTAL: THE PERIODIC TABLE – PALLADIUM. (n.d.). C&EN: IT’S ELEMENTAL: THE PERIODIC TABLE – PALLADIUM. https://pubsapp.acs.org/cen/80th/palladium.html?
