This is a SUPER easy guide on Actinium element.
In fact, the table mentioned below is the perfect information box (Which gives you every single detail about the Actinium element in Periodic table.)
So if you want to know anything about Actinium element, then this guide is for you.
Let’s finish this very quickly.
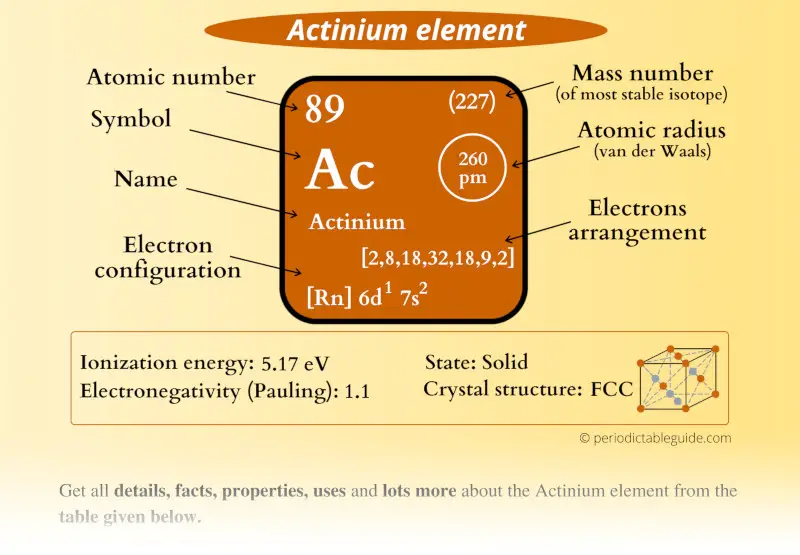
Actinium Element (Ac) Information
| Appearance | Silvery white appearance |
| State (at STP) | Solid |
| Position in Periodic table | 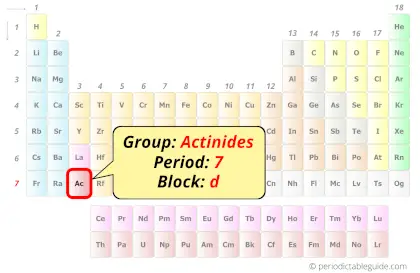 Group: actinides, Period: 7, Block: d |
| Category | 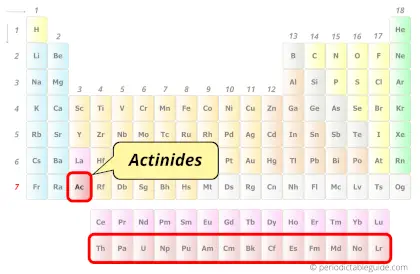 Actinides |
| Atomic number or Protons | 89 |
| Neutrons | 138 |
| Electrons | 89 |
| Symbol | Ac |
| Atomic mass of Actinium (most stable isotope) | 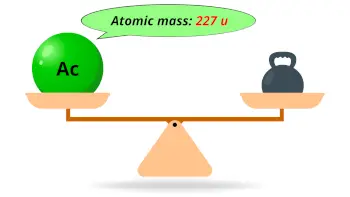 227 u |
| Electrons arrangement or Bohr model | 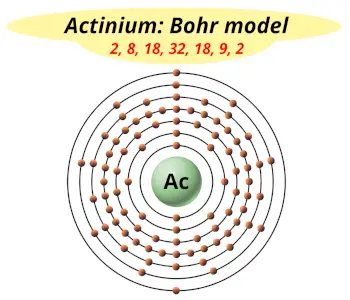 2, 8, 18, 32, 18, 9, 2 |
| Electronic configuration | [Rn] 6d1 7s2 |
| Atomic radius | 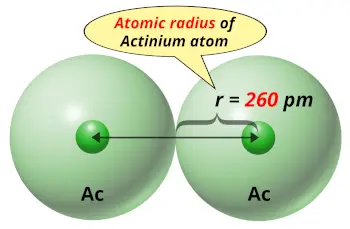 260 picometers (van der Waals radius) |
| 1st Ionization energy | 5.17 eV |
| Electronegativity | 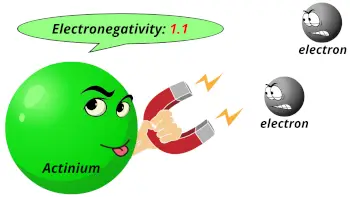 1.1 (Pauling scale) |
| Crystal structure | 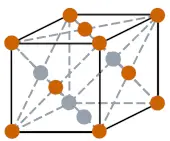 FCC (face centered cubic) |
| Density | 10.07 g/cm3 |
| Main isotope | 227Ac |
| Who discovered Actinium and when? |  Friedrich Oskar Giesel (in 1902) |
| CAS number | 7440-34-8 |
Actinium in Periodic table
Actinium element is in period 7 of the Periodic table. Actinium is the d-block element and it belongs to actinides group.
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba | La* | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| *Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| **Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
←Move to: Radium (Ra) element – Periodic Table
→Move to: Thorium (Th) element – Periodic Table
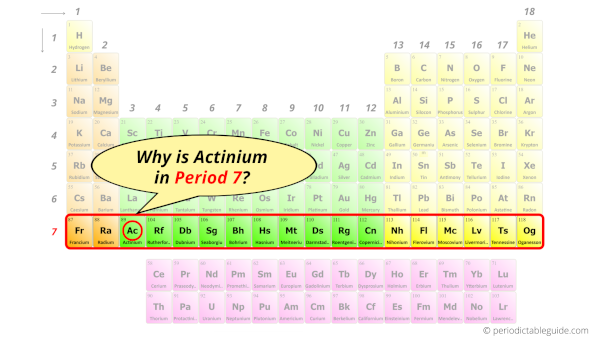
Why is Actinium in Period 7?
Let me ask you a question.
How many shells does actinium have?
It’s 7. Right?
You have already seen the bohr model of actinium atom in the above table.
From the Bohr model, it can be found that the number of orbits or shells in actinium is 7. Hence, as actinium has 7 orbits, it lies in period 7 of the Periodic table.
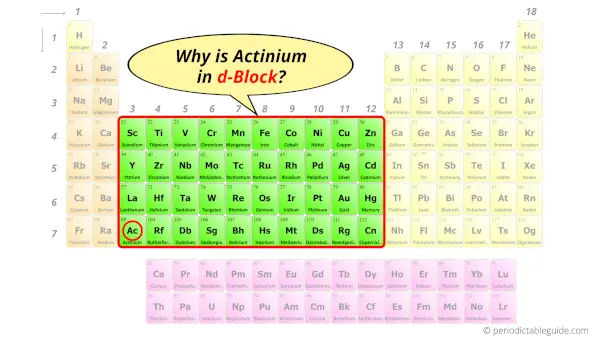
Why is Actinium in d-block?
Before knowing this reason, first of all I want to ask you a simple question.
How can you determine the blocks-wise position of elements?
The simple answer: The elements will lie in the s, p, d or f block will completely depend upon the subshell in which the last electron will enter.
For example; the electron configuration of actinium is [Rn] 7s2 6d1.
So the last electron of actinium enters the d-subshell or d-orbital.
Hence, actinium is the d-block element.
5 Interesting facts about Actinium
Interesting facts about actinium element are mentioned below.
- The word actinium was derived from the Greek word “aktis”, which means beam.
- Actinium was discovered by Friedrich Oskar Giesel in 1902.
- Actinium has many isotopes and all those isotopes are radioactive.
- Actinium naturally occurs in uranium ore.
- Actinium is around 150 times more radioactive than radium element.
Properties of Actinium
The physical and chemical properties of actinium element are mentioned below.
Physical properties of Actinium
Physical properties of actinium are mentioned below.
- Actinium is a radioactive element having a silvery white appearance.
- In a dark room, actinium glows blue in color.
- The atomic mass of the most stable isotope of actinium is 227 u and its density is 10.07 g/cm3.
- The crystal structure of Actinium is FCC (i.e face centered cubic).
Chemical properties of Actinium
Chemical properties of actinium are mentioned below.
- In the moist air, actinium reacts with oxygen and forms a thin coating of actinium oxide on it.
- Actinium is a radioactive element and it is hazardous to human health due to its radioactivity.
- If actinium enters the human body, then it will get deposited into bones and lever, which damages the cells due to radioactive decay. This causes bone cancer and other illnesses.
- The most common oxidation state of actinium is +3.
Uses of Actinium
Actinium is generally used for research work. But apart from this, actinium is also a good source of neutrons. It can also be used in treatment of cancer.
Moreover due to its radioactive nature, it is not commercially used.
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
External resources:
- Actinium – Element information, properties and uses | Periodic Table. (n.d.). Actinium – Element Information, Properties and Uses | Periodic Table. https://www.rsc.org/periodic-table/element/89/actinium
- Actinium – Wikipedia. (2011, June 2). Actinium – Wikipedia. https://en.wikipedia.org/wiki/Actinium
- P. (n.d.). Actinium | Ac (Element) – PubChem. Actinium | Ac (Element) – PubChem. https://pubchem.ncbi.nlm.nih.gov/element/Actinium
- It’s Elemental – The Element Actinium. (n.d.). It’s Elemental – the Element Actinium. https://education.jlab.org/itselemental/ele089.html
- C&EN: IT’S ELEMENTAL: THE PERIODIC TABLE – ACTINIUM. (n.d.). C&EN: IT’S ELEMENTAL: THE PERIODIC TABLE – ACTINIUM. http://pubsapp.acs.org/cen/80th/actinium.html?
