This is a SUPER easy guide on Manganese element.
In fact, the table mentioned below is the perfect information box (Which gives you every single detail about the Manganese element in Periodic table.)
So if you want to know anything about manganese element, then this guide is for you.
Let’s finish this very quickly.
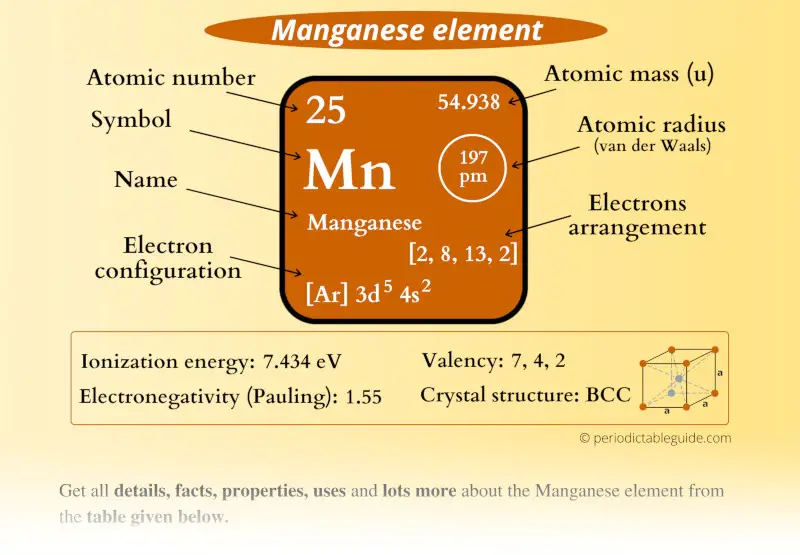
Manganese Element (Mn) Information
| Appearance |  Silvery white metallic |
| State (at STP) | Solid |
| Position in Periodic table | 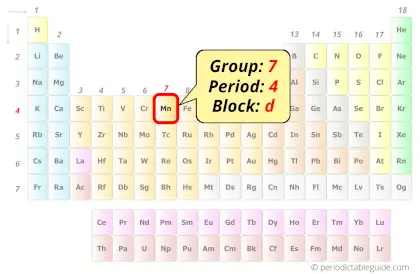 Group: 7, Period: 4, Block: d |
| Category | 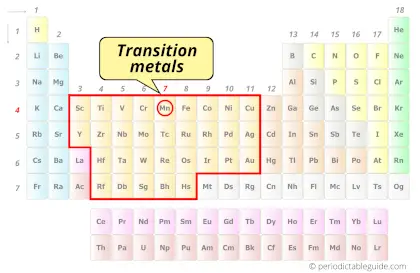 Transition metals |
| Atomic number or Protons | 25 |
| Neutrons | 30 |
| Electrons | 25 |
| Symbol | Mn |
| Atomic mass | 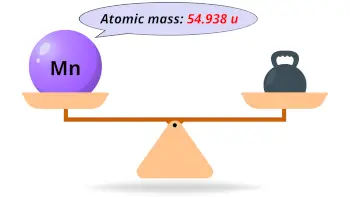 54.938 u |
| Electrons arrangement or Bohr model | 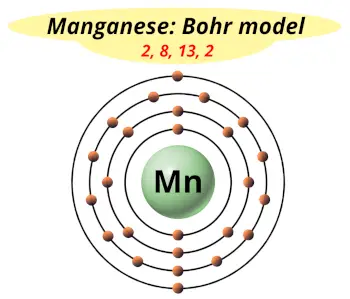 2, 8, 13, 2 |
| Electronic configuration | [Ar] 3d5 4s2 |
| Atomic radius | 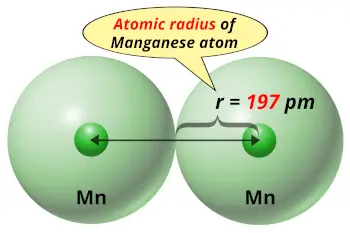 197 picometers (van der Waals radius) |
| 1st Ionization energy | 7.434 eV |
| Electronegativity | 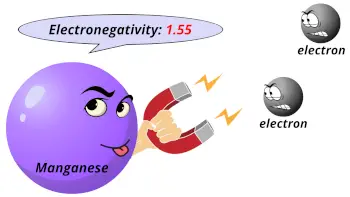 1.55 (Pauling scale) |
| Crystal structure | 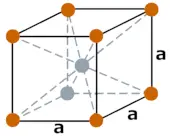 BCC (Body centered cubic) |
| Melting point | 1519 K or 1246 °C or 2275 °F |
| Boiling point | 2334 K or 2061 °C or 3742 °F |
| Density | 7.47 g/cm3 |
| Main isotope | 55Mn |
| Who discovered Manganese and when? |  Carl Wilhelm Scheele in 1774 |
| CAS number | 7439-96-5 |
Manganese in Periodic table
Manganese element is in group 7 and period 4 of the Periodic table. Manganese is the d-block element and it belongs to transition metals group.
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba | La* | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| *Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| **Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
←Move to: Chromium (Cr) element – Periodic Table
→Move to: Iron (Fe) element – Periodic Table
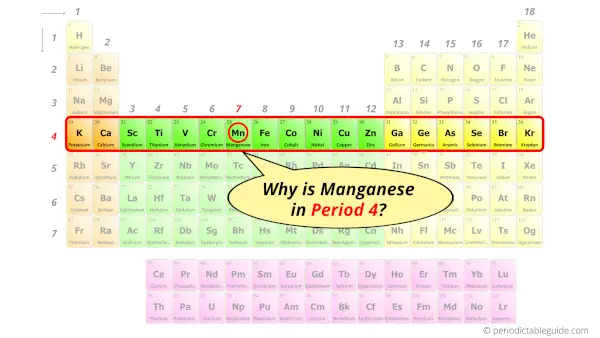
Why is Manganese in Period 4?
Let me ask you a question.
How many shells does manganese have?
It’s 4. Right?
You have already seen the bohr model of manganese atom in the above table.
From the Bohr model, it can be found that the number of orbits or shells in manganese is 4. Hence, as manganese has 4 orbits, it lies in period 4 of the Periodic table.
Why is Manganese in d-block?
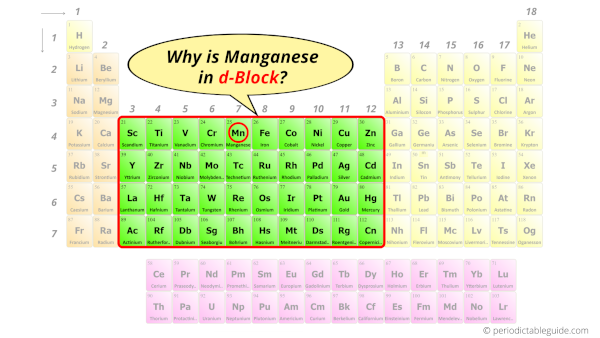
Before knowing this reason, first of all I want to ask you a simple question.
How can you determine the blocks-wise position of elements?
The simple answer: The elements will lie in the s, p, d or f block will completely depend upon the subshell in which the last electron will enter.
For example; the electron configuration of manganese is [Ar] 4s2 3d5.
So the last electron of manganese enters the d-subshell or d-orbital.
Hence, manganese is the d-block element.
Is Manganese a Transition Metal? Why?
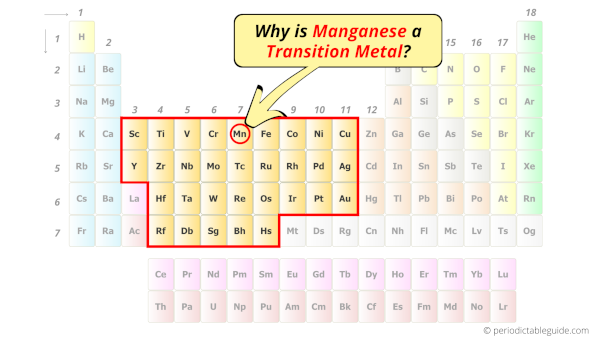
Yes, Manganese is a transition metal because it has incompletely filled d-orbital in its ground state.
Let me explain the exact meaning of this.
According to the definition of transition metals;
The element should compulsorily have incomplete d-orbitals, either in their ground state (M) or most common oxidation states (M1+, M2+, etc) then only they are called transition metals.
Now, the ground state of Manganese means its normal state in which it has neither gained nor lost any electron/s.
So the ground state of Manganese is Mn.
And the ground state electronic configuration of Manganese is [Ar] 4s2 3d5.
In this state, if we see the electron configuration of Manganese, then it possesses incomplete d-orbitals.
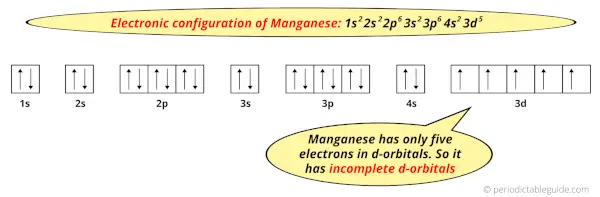
Because, there are only five electrons in the d-orbitals.
In order to have the complete d-orbitals, there must be 10 electrons in it.
But in the ground state electronic configuration of manganese, you can see that it has only 5 electrons in d-orbitals.
Thus, Manganese has incomplete d-orbitals.
And hence, as Manganese has incomplete d-orbitals, it is considered as a transition metal.
Also see: Location of transition metals on periodic table (Image)
5 Interesting facts about Manganese
Interesting facts about manganese element are mentioned below.
- According to USGS, around 85 to 90% manganese is used in production of steels.
- Around 0.1% (by weight) of the earth’s crust consists of manganese element making it the 12th most abundant element in the earth’s crust.
- Magnesium is also required for the human body to perform some metabolic functions.
- The hardness of manganese is more than iron, but it is a brittle metal than iron.
- South Africa is a leading country which has around 75% of the manganese resources.
Properties of Manganese
The physical and chemical properties of manganese element are mentioned below.
Physical properties of Manganese
Physical properties of manganese are mentioned below.
- Manganese is a transition metal having a silvery white metallic appearance.
- Manganese is shiny when polished, but it tarnishes when exposed to air.
- The melting point of Manganese is 1246 °C and its boiling point is 2061 °C.
- Atomic mass of manganese is 54.938 u and its density is 7.47 g/cm3.
- Manganese is a strong but brittle metal. And hence it is not malleable.
Chemical properties of Manganese
Chemical properties of manganese are mentioned below.
- Manganese is a fairly reactive element, and so it does not exist in free state. But it is always found as a compound with other elements.
- Manganese has incomplete d-orbitals, hence it is classified as a transition metal on the periodic table.
- Manganese has many isotopes, but out of these 55Mn has the maximum abundance (almost 100%).
- Pure manganese is chemically reactive which gets corroded when it comes in contact with water.
Uses of Manganese
Uses of manganese are mentioned below.
- Manganese is used as an alloying element in the production of steel, glass and other materials.
- Manganese is an essential mineral element used by the human body for some metabolic functions. And the manganese is obtained from foods like spinach, eggs, grains, rice, nuts, etc.
- Manganese is also used in alloys of aluminum that are used to manufacture cans for storing beverages.
Free Gift for you: Interactive Periodic Table
Let me tell you how this Interactive Periodic Table will help you in your studies.
1). You can effortlessly find every single detail about the elements from this single Interactive Periodic table.
2). You will get the detailed information about the periodic table which will convert a newbie into pro.
3). You will also get the HD images of the Periodic table (for FREE).
Checkout Interactive Periodic table and download it’s high resolution image now (It’s FREE)
External resources:
- Manganese. (n.d.). Manganese. https://webbook.nist.gov/cgi/inchi/InChI%3D1S/Mn
- Manganese – Element information, properties and uses | Periodic Table. (n.d.). Manganese – Element Information, Properties and Uses | Periodic Table. https://www.rsc.org/periodic-table/element/25/manganese
- Manganese – Wikipedia. (2019, September 7). Manganese – Wikipedia. https://en.wikipedia.org/wiki/Manganese
- P. (n.d.). Manganese | Mn (Element) – PubChem. Manganese | Mn (Element) – PubChem. https://pubchem.ncbi.nlm.nih.gov/element/Manganese
- It’s Elemental – The Element Manganese. (n.d.). It’s Elemental – the Element Manganese. https://education.jlab.org/itselemental/ele025.html