This is a SUPER easy guide on Neodymium element.
In fact, the table mentioned below is the perfect information box (Which gives you every single detail about the Neodymium element in Periodic table.)
So if you want to know anything about Neodymium element, then this guide is for you.
Let’s dive right into it!
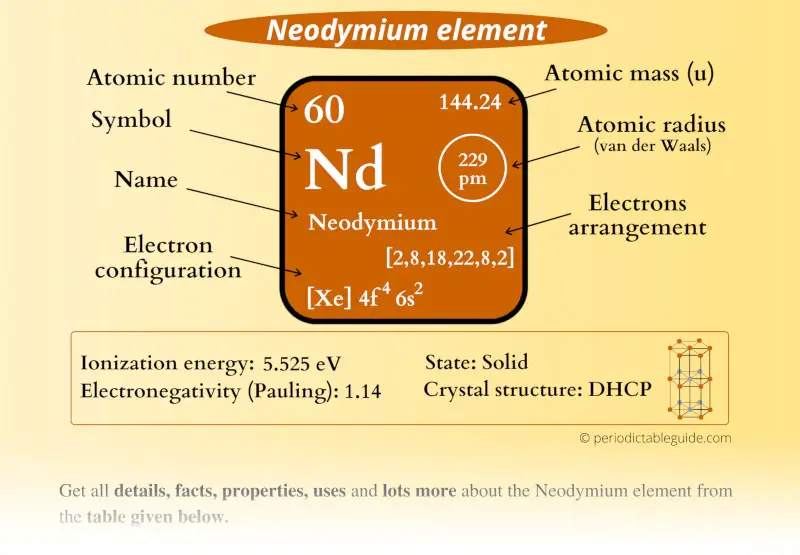
Neodymium Element (Nd) Information
| Appearance |  Silvery white metallic |
| State (at STP) | Solid |
| Position in Periodic table | 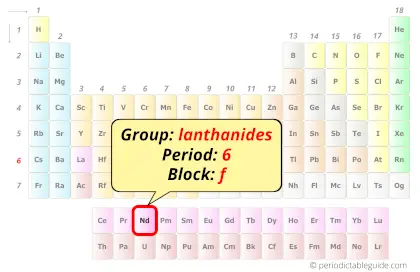 Group: lanthanides, Period: 6, Block: f |
| Category | 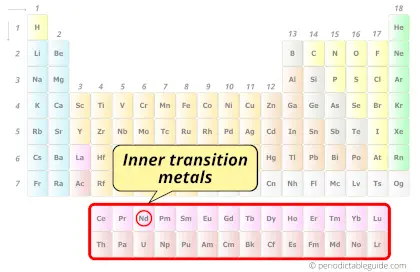 Inner transition metals |
| Atomic number or Protons | 60 |
| Neutrons | 84 |
| Electrons | 60 |
| Symbol | Nd |
| Atomic mass | 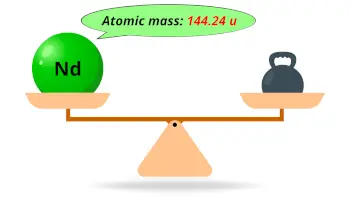 144.24 u |
| Electrons arrangement or Bohr model | 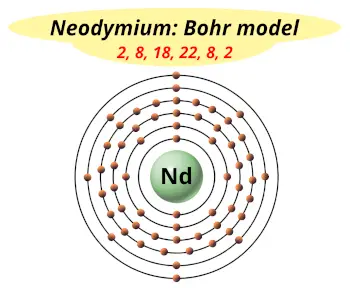 2, 8, 18, 22, 8, 2 |
| Electronic configuration | [Xe] 4f4 6s2 |
| Atomic radius | 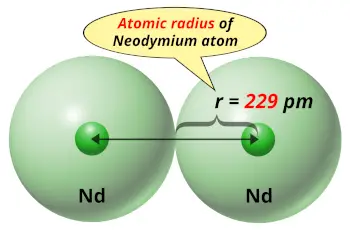 229 picometers (van der Waals radius) |
| 1st Ionization energy | 5.525 eV |
| Electronegativity | 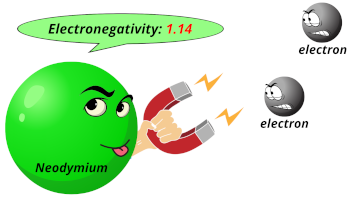 1.14 (Pauling scale) |
| Crystal structure | 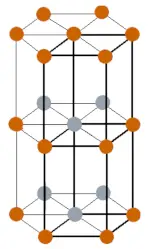 Double hexagonal close packed |
| Melting point | 1297 K or 1024 °C or 1875 °F |
| Boiling point | 3347 K or 3074 °C or 5565 °F |
| Density | 7.01 g/cm3 |
| Main isotope | 142Nd |
| Who discovered Neodymium and when? |  Carl Auer von Welsbach in 1885 |
| CAS number | 7440-00-8 |
Neodymium in Periodic table
Neodymium element is in period 6 and in lanthanide group of the Periodic table. Neodymium is the f-block element and it belongs to inner transition metals group.
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba | La* | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| *Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| **Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
←Move to: Praseodymium (Pr) element – Periodic Table
→Move to: Promethium (Pm) element – Periodic Table
Why is Neodymium in Period 6?
Let me ask you a question.
How many shells does neodymium have?
It’s 6. Right?
You have already seen the bohr model of neodymium atom in the above table.
From the Bohr model, it can be found that the number of orbits or shells in neodymium is 6. Hence, as neodymium has 6 orbits, it lies in period 6 of the Periodic table.
Why is Neodymium in f-block?
Before knowing this reason, first of all I want to ask you a simple question.
How can you determine the blocks-wise position of elements?
The simple answer: The elements will lie in the s, p, d or f block will completely depend upon the subshell in which the last electron will enter.
For example; the electron configuration of neodymium is [Xe] 6s2 4f4.
So the last electron of neodymium enters the f-subshell or f-orbital.
Hence, neodymium is the f-block element.
5 Interesting facts about Neodymium
Interesting facts about neodymium element are mentioned below.
- The name neodymium was derived from the two Greek words “neos” and “dymium”. “Neos” means “new” and “dymium” means “twin”.
- Neodymium was discovered by Carl Auer von Welsbach in 1885.
- Neodymium is a rare earth metal, but it is not actually rare in quantity. The fact is that it is spread evenly on the earth and it is very difficult to find this element at one place on the earth. Thus it is rare in the context of available resources.
- Out of all the rare earth metals on periodic table, neodymium is the 2nd most abundant rare earth metal.
- The abundance of neodymium in the earth’s crust is 33 ppm by weight.
Properties of Neodymium
The physical and chemical properties of neodymium element are mentioned below.
Physical properties of Neodymium
Physical properties of neodymium are mentioned below.
- Neodymium is a solid metal having a Silvery white metallic appearance.
- The melting point of neodymium is 1024 °C and its boiling point is 3074 °C.
- The atomic mass of neodymium is 144.24 u and its density is 7.01 g/cm3.
- Neodymium has a double hexagonal close packed crystal structure.
- Neodymium has many isotopes. Out of them, the most abundant isotope is 142Nd (having an abundance of 27.2%).
Chemical properties of Neodymium
Chemical properties of neodymium are mentioned below.
- Neodymium is a chemically reactive metal and hence it is not found in free state. It is always found as a compound with other metals in the earth’s crust.
- Neodymium reacts with the atmospheric oxygen and gets oxidized.
- As neodymium is chemically reactive, it is necessary to keep it under mineral oil or any other protective atmosphere.
- The electron configuration of neodymium is [Xe] 6s2 4f4, which shows that the last electron enters the f-orbital. Hence neodymium is a f-block element.
- The first ionization energy of neodymium is 5.525 eV.
- The electronegativity of neodymium is 1.14 on the Pauling scale.
Uses of Neodymium
Uses of neodymium are mentioned below.
- Neodymium is used in the manufacturing of colored glass. The color of these glass ranges from violet, red and grey.
- Neodymium is used in making magnets. The neodymium magnets are an alloy of neodymium, boron and iron.
- Neodymium is also used in making flints for lighters which produce spark.
- Neodymium is also used as a coloring agent in enamels.
- Neodymium is used as a crystal in Nd:YAG lasers. Nd:YAG stands for Neodymium doped Yttrium Aluminum Garnet.
Free Gift for you: Interactive Periodic Table
Let me tell you how this Interactive Periodic Table will help you in your studies.
1). You can effortlessly find every single detail about the elements from this single Interactive Periodic table.
2). You will get the detailed information about the periodic table which will convert a newbie into pro.
3). You will also get the HD images of the Periodic table (for FREE).
Checkout Interactive Periodic table and download it’s high resolution image now (It’s FREE)
External resources:
- Neodymium – Element information, properties and uses | Periodic Table. (n.d.). Neodymium – Element Information, Properties and Uses | Periodic Table. https://www.rsc.org/periodic-table/element/60/neodymium
- Neodymium – Wikipedia. (2012, August 16). Neodymium – Wikipedia. https://en.wikipedia.org/wiki/Neodymium
- Neodymium – American Chemical Society. (n.d.). American Chemical Society. https://www.acs.org/greenchemistry/research-innovation/endangered-elements/neodymium.html
- P. (n.d.). Neodymium | Nd (Element) – PubChem. Neodymium | Nd (Element) – PubChem. https://pubchem.ncbi.nlm.nih.gov/element/Neodymium
- It’s Elemental – The Element Neodymium. (n.d.). It’s Elemental – the Element Neodymium. https://education.jlab.org/itselemental/ele060.html
- Periodic Table of Elements: Los Alamos National Laboratory. (n.d.). Periodic Table of Elements: Los Alamos National Laboratory. https://periodic.lanl.gov/60.shtml