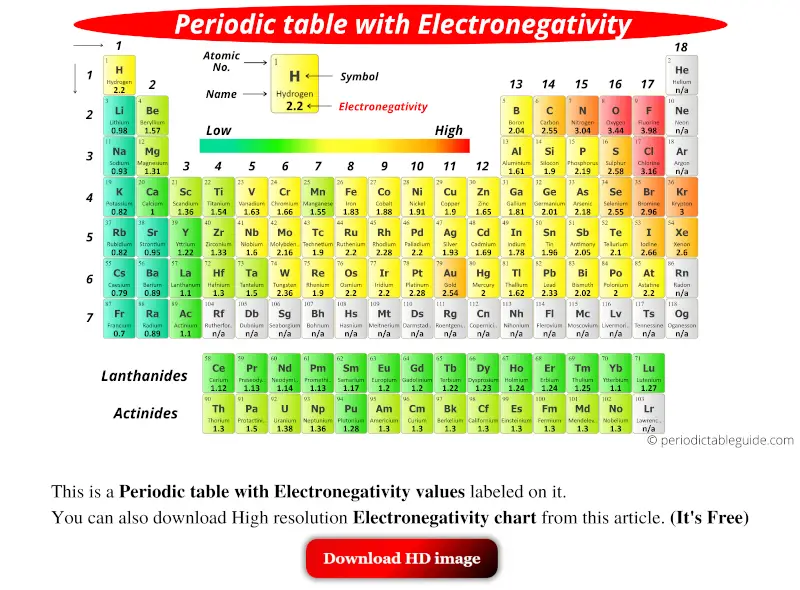
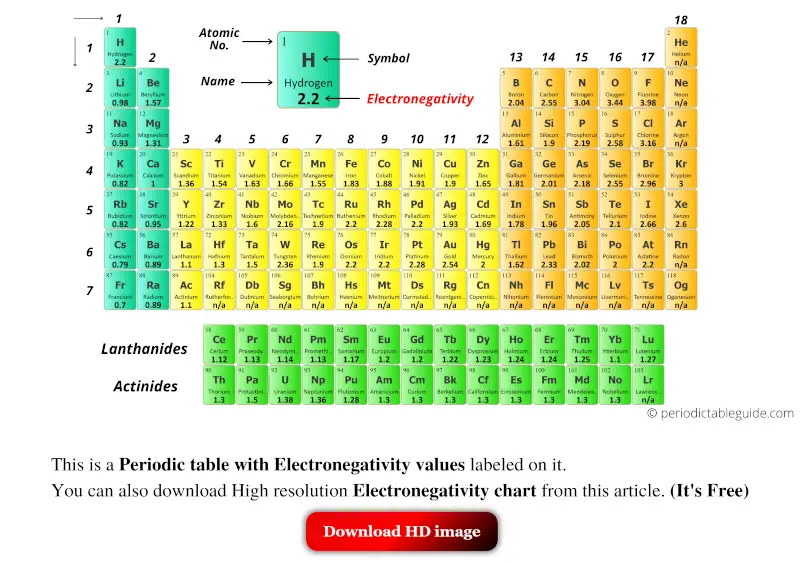
The above image clearly shows you the Periodic table with Electronegativity Values labeled on it.
You can also get the HD printable Periodic table with Electronegativity, from this article only. (Downloading link given below)
There are many more labeled Periodic Table of electronegativity given below.
But before that, just have a look at the concept of Electronegativity.
Electronegativity: Electronegativity is a tendency to attract the shared pair of electrons.
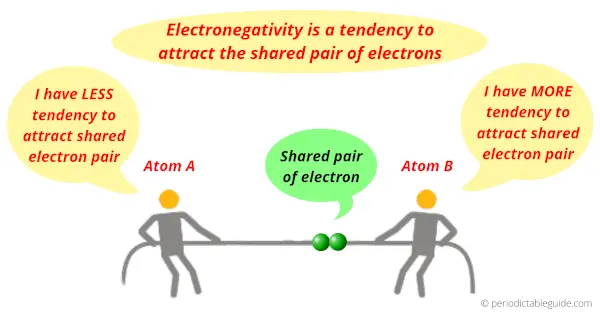
The atoms having more tendency to attract the electron pair are more electronegative.
And the atoms having less tendency to attract the electron pair are less electronegative.
In the above image, you can see that Atom B has more tendency to attract the shared pair of electrons, hence Atom B is more electronegative than Atom A.
(Note: Electronegativity has no unit. Linus Pauling was a scientist who designed a scale of electronegativity that ranks the elements with respect to each other. And this scale is known as Pauling electronegativity scale.)
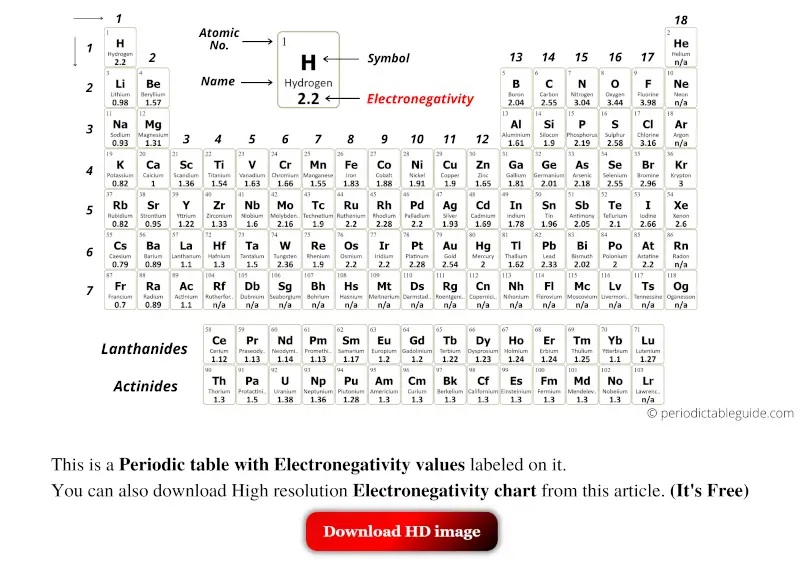
Let’s see few Periodic tables of elements with Electronegativity values labeled on it.
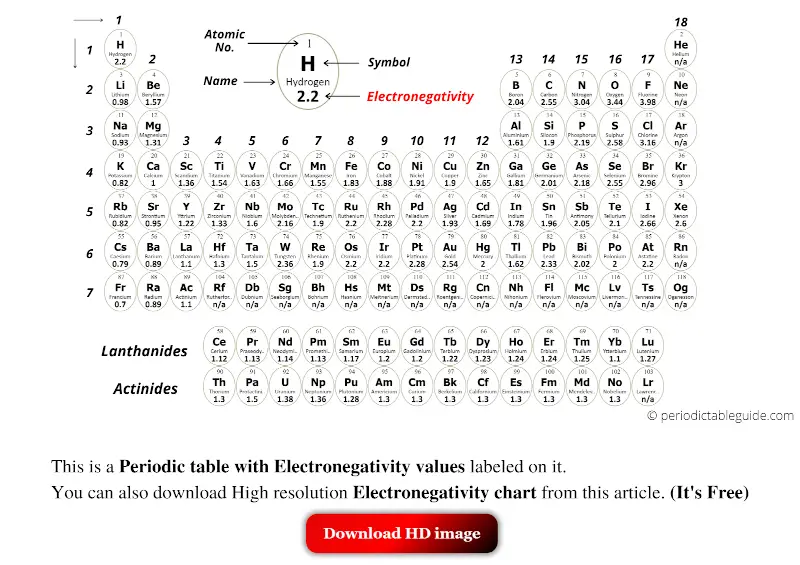
Periodic table with Electronegativity (Circular tile)
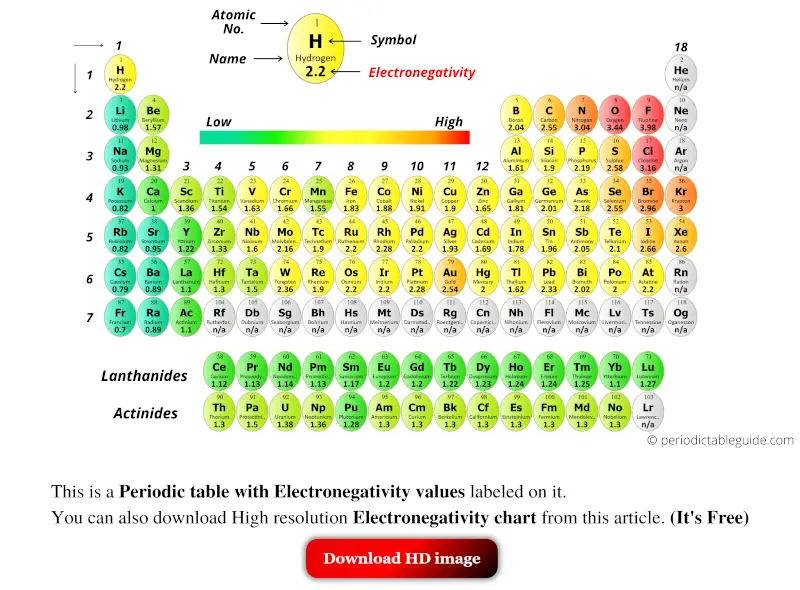
Periodic table with Electronegativity and groups
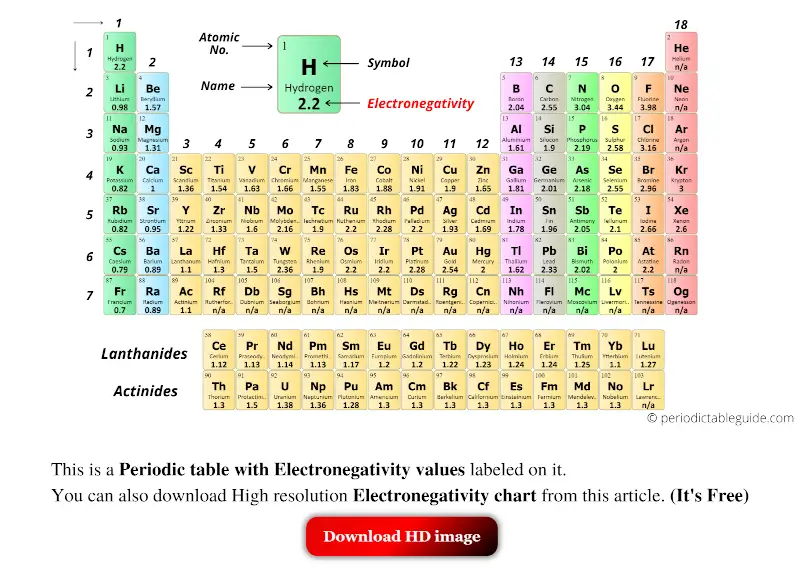
Periodic table with Electronegativity and blocks
The elements in blue colour are the s-block elements.
Orange colored elements are p-block elements.
Yellow colored elements are d-block elements and Green colored elements are the f-block elements.
Black and White
With circular tile (Black and white)
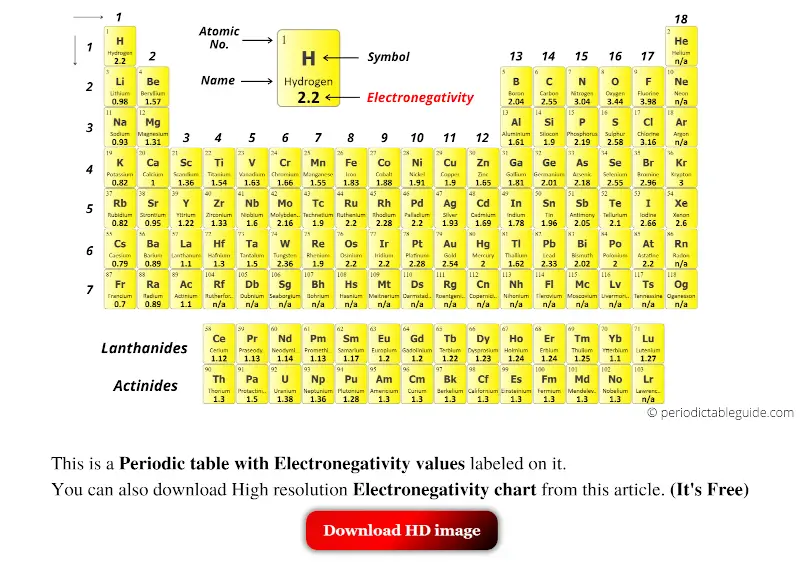
Yellow colored Periodic table
Electronegativity of Elements (List)
List of elements with their electronegativity values are given below.
| Atomic number | Elements | Electronegativity |
|---|---|---|
| 1 | Hydrogen | 2.2 |
| 2 | Helium | N/A |
| 3 | Lithium | 0.98 |
| 4 | Beryllium | 1.57 |
| 5 | Boron | 2.04 |
| 6 | Carbon | 2.55 |
| 7 | Nitrogen | 3.04 |
| 8 | Oxygen | 3.44 |
| 9 | Fluorine | 3.98 |
| 10 | Neon | N/A |
| 11 | Sodium | 0.93 |
| 12 | Magnesium | 1.31 |
| 13 | Aluminum | 1.61 |
| 14 | Silicon | 1.9 |
| 15 | Phosphorus | 2.19 |
| 16 | Sulfur | 2.58 |
| 17 | Chlorine | 3.16 |
| 18 | Argon | N/A |
| 19 | Potassium | 0.82 |
| 20 | Calcium | 1 |
| . | . | . |
| . | . | . |
| . | . | . |
These are the electronegativities of first 20 Elements of the periodic table.
But if you want to see the electronegativities of all the 118 elements, then visit: Electronegativity chart of all the elements. (Make sure you visit this, because I have mentioned the electronegativities along with the images for each element).
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
External links:
Electronegativity of elements
