This is a SUPER easy guide on Erbium element.
In fact, the table mentioned below is the perfect information box (Which gives you every single detail about the Erbium element in Periodic table.)
So if you want to know anything about Erbium element, then this guide is for you.
Let’s finish this very quickly.
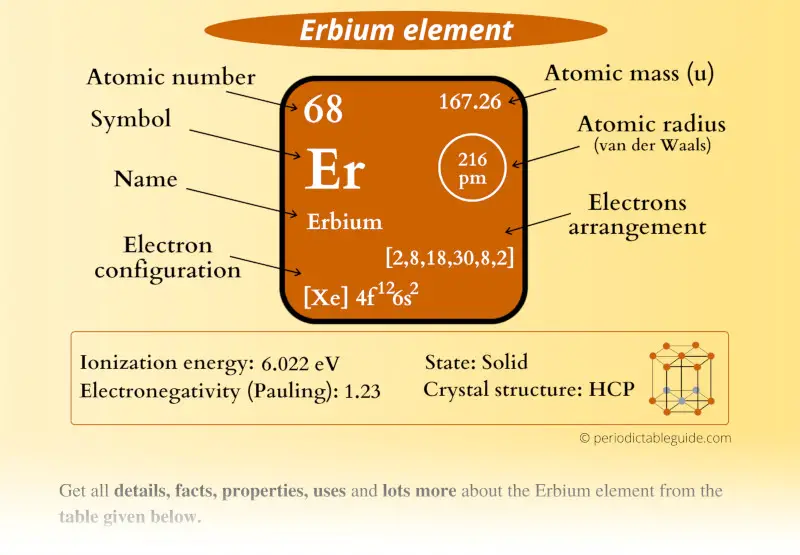
Erbium Element (Er) Information
| Appearance |  Silvery white metallic surface |
| State (at STP) | Solid |
| Position in Periodic table | 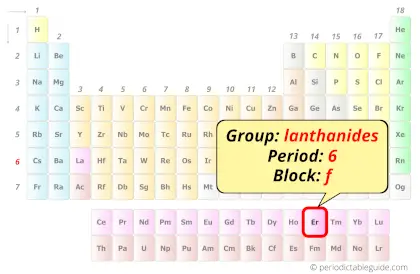 Group: lanthanides, Period: 6, Block: f |
| Category | 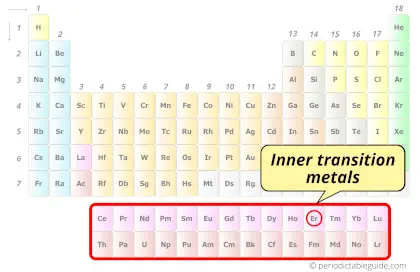 Inner transition metals |
| Atomic number or Protons | 68 |
| Neutrons | 99 |
| Electrons | 68 |
| Symbol | Er |
| Atomic mass | 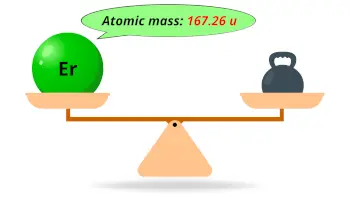 167.26 u |
| Electrons arrangement or Bohr model | 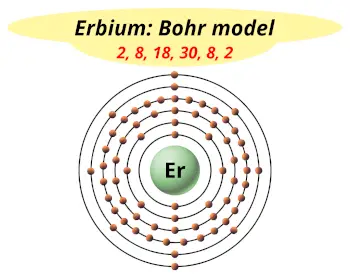 2, 8, 18, 30, 8, 2 |
| Electronic configuration | [Xe] 4f12 6s2 |
| Atomic radius | 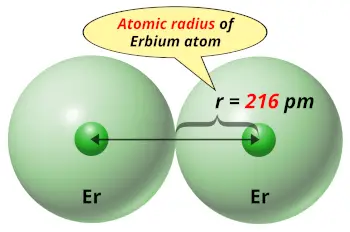 216 picometers (van der Waals radius) |
| 1st Ionization energy | 6.022 eV |
| Electronegativity | 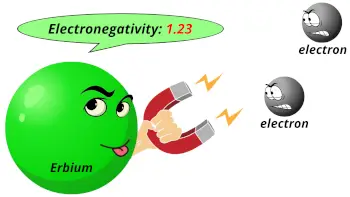 1.23 (Pauling scale) |
| Crystal structure | 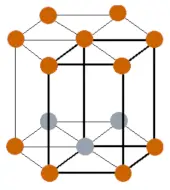 HCP (Hexagonal close packed) |
| Melting point | 1802 K or 1529 °C or 2784 °F |
| Boiling point | 3141 K or 2868 °C or 5194 °F |
| Density | 9.06 g/cm3 |
| Main isotope | 166Er |
| Who discovered Erbium and when? |  Carl Gustaf Mosander (in 1843) |
| CAS number | 7440-52-0 |
Erbium in Periodic table
Erbium element is in period 6 and in lanthanide group of the Periodic table. Erbium is the f-block element and it belongs to inner transition metals group.
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba | La* | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| *Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| **Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
←Move to: Holmium (Ho) element – Periodic Table
→Move to: Thulium (Tm) element – Periodic Table
Why is Erbium in Period 6?
Let me ask you a question.
How many shells does erbium have?
It’s 6. Right?
You have already seen the bohr model of erbium atom in the above table.
From the Bohr model, it can be found that the number of orbits or shells in erbium is 6. Hence, as erbium has 6 orbits, it lies in period 6 of the Periodic table.
Why is Erbium in f-block?
Before knowing this reason, first of all I want to ask you a simple question.
How can you determine the blocks-wise position of elements?
The simple answer: The elements will lie in the s, p, d or f block will completely depend upon the subshell in which the last electron will enter.
For example; the electron configuration of erbium is [Xe] 6s2 4f12.
So the last electron of erbium enters the f-subshell or f-orbital.
Hence, erbium is the f-block element.
5 Interesting facts about Erbium
Interesting facts about erbium element are mentioned below.
- The name “Erbium” came from the name of a small village “Ytterby” of Sweden.
- The erbium element was discovered by Carl Gustaf Mosander (in 1843).
- Erbium is not found free in nature, but it is found from its minerals like gadolinite, euxenite, etc.
- The Erbium element was discovered by Carl Gustaf Mosander (in 1843).
- The concentration of erbium in the earth’s crust is approximately 3 ppm by weight.
Properties of Erbium
The physical and chemical properties of erbium element are mentioned below.
Physical properties of Erbium
Physical properties of erbium are mentioned below.
- Erbium element is soft and malleable and it has a silvery white metallic surface.
- The atomic mass of erbium is 167.26 u and its density is 9.06 g/cm3.
- The melting point and boiling point of erbium are 1529 °C and 2868 °C respectively.
- The crystal structure of erbium is HCP (Hexagonal close packed).
- Erbium has many isotopes (including natural isotopes as well as synthetic isotopes). But out of those isotopes, the most abundant isotope is 166Er (having an abundance of approximately 33.5 %).
Chemical properties of Erbium
Chemical properties of erbium are mentioned below.
- Erbium metal is much stable in air at room temperature, and it does not oxidize easily at room temperature.
- Erbium reacts slowly with water and it also dissolves in acids.
- The salts of erbium are light pink in color and they exist as Er3+ ions in its compounds.
Uses of Erbium
Uses of erbium are mentioned below.
- Erbium has a good neutron absorbing capacity. Hence it is used as a control rod in nuclear reactors.
- The oxide of the erbium element is used to give pink tint in glasses.
- The hardness of metals can be reduced by adding a small proportion of erbium in it.
- Erbium has some medical applications too. It is used in lasers to treat skin problems.
Free Gift for you: Interactive Periodic Table
Let me tell you how this Interactive Periodic Table will help you in your studies.
1). You can effortlessly find every single detail about the elements from this single Interactive Periodic table.
2). You will get the detailed information about the periodic table which will convert a newbie into pro.
3). You will also get the HD images of the Periodic table (for FREE).
Checkout Interactive Periodic table and download it’s high resolution image now (It’s FREE)
External resources:
- Atomic Data for Erbium (Er). (n.d.). Atomic Data for Erbium (Er). https://physics.nist.gov/PhysRefData/Handbook/Tables/erbiumtable1.htm
- Erbium – Wikipedia. (2009, May 3). Erbium – Wikipedia. https://en.wikipedia.org/wiki/Erbium
- Erbium – Element information, properties and uses | Periodic Table. (n.d.). Erbium – Element Information, Properties and Uses | Periodic Table. https://www.rsc.org/periodic-table/element/68/erbium
- P. (n.d.). Erbium | Er (Element) – PubChem. Erbium | Er (Element) – PubChem. https://pubchem.ncbi.nlm.nih.gov/element/Erbium
- It’s Elemental – The Element Erbium. (n.d.). It’s Elemental – the Element Erbium. https://education.jlab.org/itselemental/ele068.html