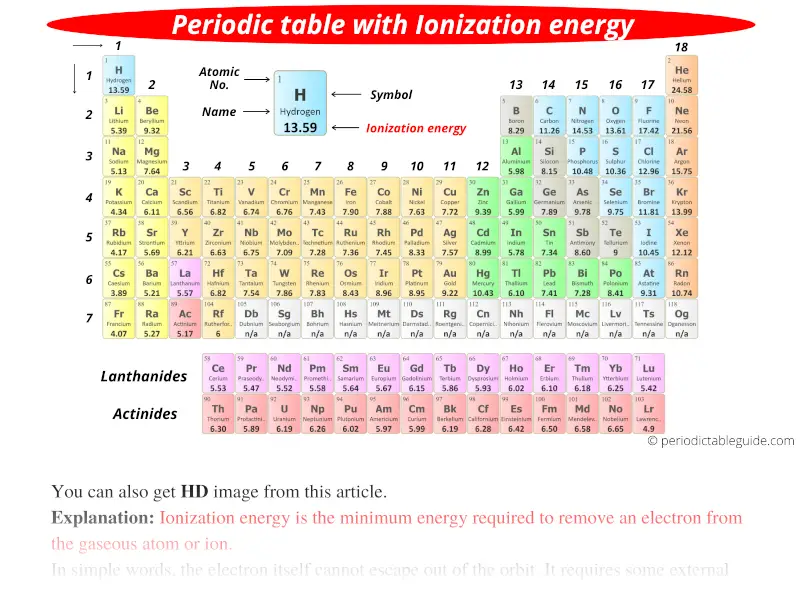
The above image clearly shows you the Periodic table with Ionization Energy Values labeled on it.
Note: The ionization energy is measured in joules (J) or electron volts (eV). The values mentioned in the above and below periodic table/s are the First Ionization energy and are given in electron volts (eV).
You can also get the HD printable Periodic table with Ionization energy, from this article only. (Downloading link given below)
There are many more labeled Periodic Table of ionization energy given below.
But before that, just have a look at the concept of ionization energy.
Ionization energy: Ionization energy is the minimum energy required to remove an electron from the gaseous atom or ion.
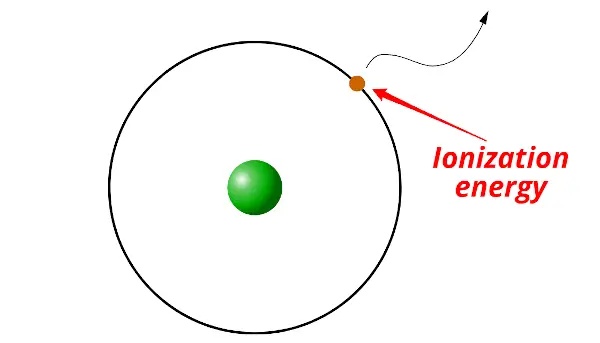
In simple words, the electron itself cannot escape out of the orbit. It requires some external energy to be supplied on it in order to escape out of the orbit. And this required energy is known as ionization energy.
These Ionization energies of all the elements are mentioned on the above periodic table.
You can see few more images of periodic tables with ionization energy labeled on it. (See below section).
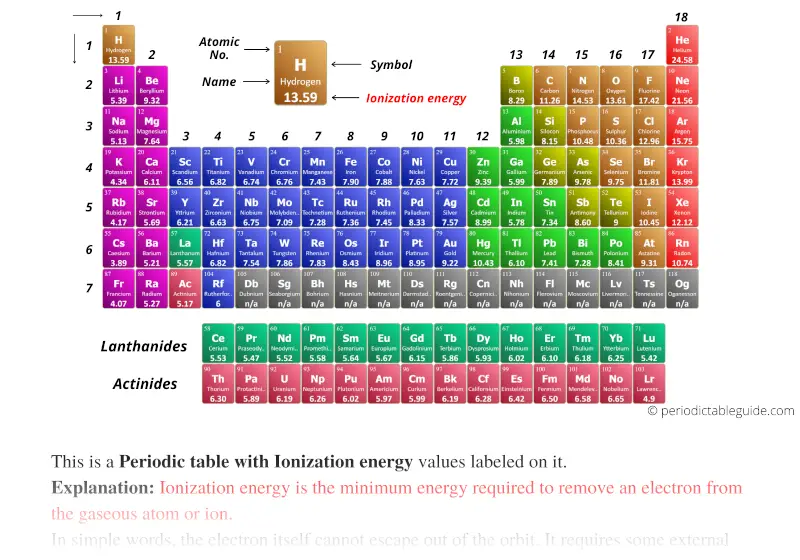
Periodic table with Ionization energy (Dark colors)
Periodic table with Ionization energy and blocks
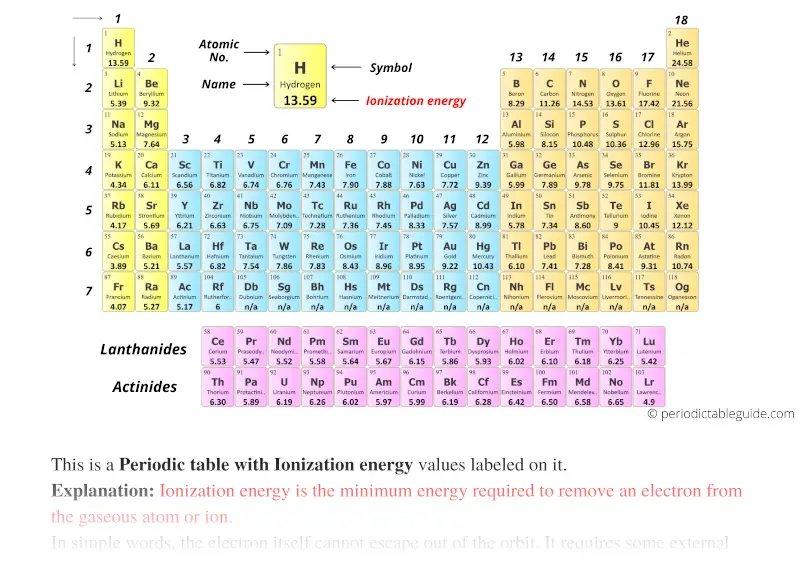
The elements in yellow colour are the s-block elements.
Orange colored elements are p-block elements.
Blue colored elements are d-block elements and purple elements are the f-block elements.
Black and White
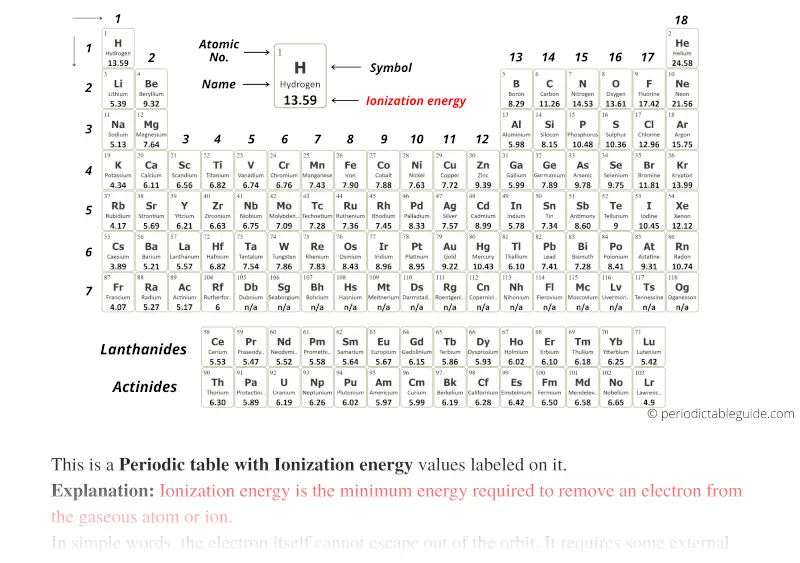
With circular tile
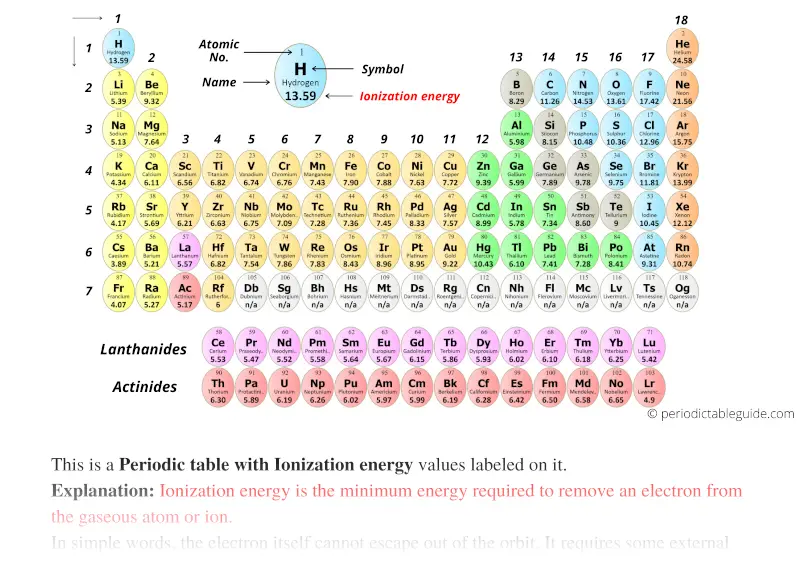
With circular tile (black and white)
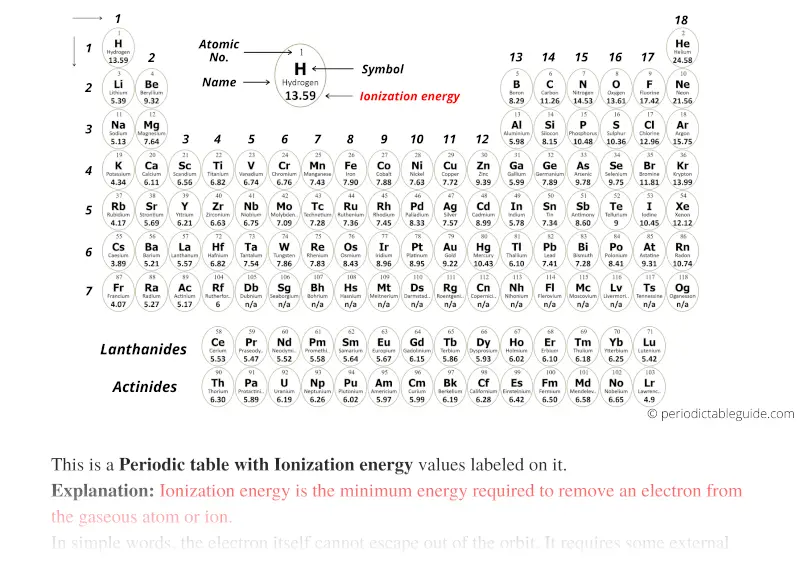
Blue colored Periodic table
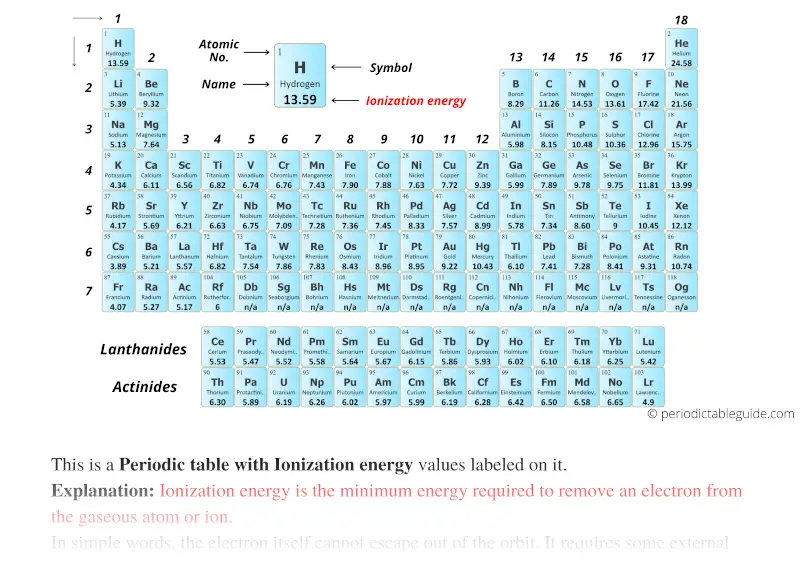
Ionization energy of Elements (List)
List of elements with their ionization energies values in eV (electron volt) are given below.
| Atomic number | Elements | First Ionization energy |
|---|---|---|
| 1 | Hydrogen | 13.59 eV |
| 2 | Helium | 24.58 eV |
| 3 | Lithium | 5.39 eV |
| 4 | Beryllium | 9.32 eV |
| 5 | Boron | 8.29 eV |
| 6 | Carbon | 11.26 eV |
| 7 | Nitrogen | 14.53 eV |
| 8 | Oxygen | 13.61 eV |
| 9 | Fluorine | 17.42 eV |
| 10 | Neon | 21.56 eV |
| 11 | Sodium | 5.13 eV |
| 12 | Magnesium | 7.64 eV |
| 13 | Aluminum | 5.98 eV |
| 14 | Silicon | 8.15 eV |
| 15 | Phosphorus | 10.48 eV |
| 16 | Sulfur | 10.36 eV |
| 17 | Chlorine | 12.96 eV |
| 18 | Argon | 15.75 eV |
| 19 | Potassium | 4.34 eV |
| 20 | Calcium | 6.11 eV |
| . | . | . |
| . | . | . |
| . | . | . |
These are the ionization energies of first 20 Elements of the periodic table.
But if you want to see the ionization energy of all the 118 elements, then visit: Ionization energy chart of all the elements. (Here you will get the first, second and third Ionization energy of all the elements in a single chart).
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
References:
Ionization energies of elements (Wikipedia)
NIST Atomic Spectra Database Ionization Energies Form
