This is a SUPER easy guide on Curium element.
In fact, the table mentioned below is the perfect information box (Which gives you every single detail about the Curium element in Periodic table.)
So if you want to know anything about Curium element, then this guide is for you.
Let’s dive right into it!
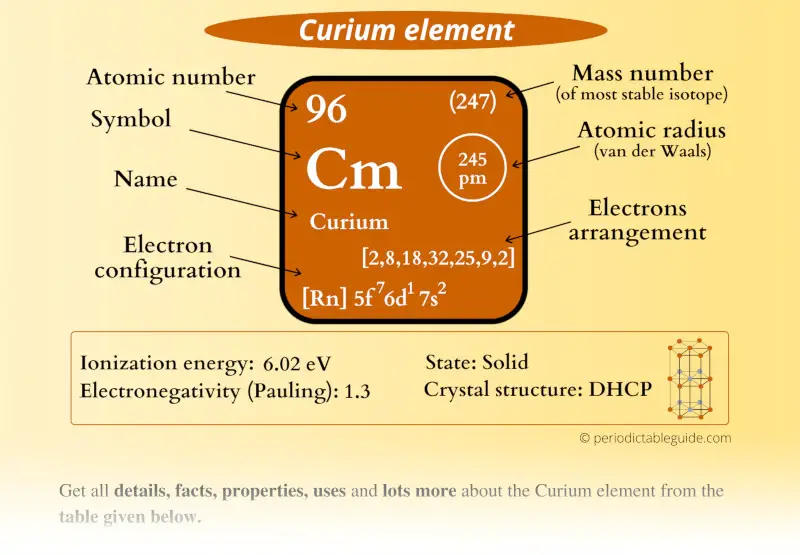
Curium Element (Cm) Information
| Appearance | Silvery metallic appearance |
| State (at STP) | Solid |
| Position in Periodic table | 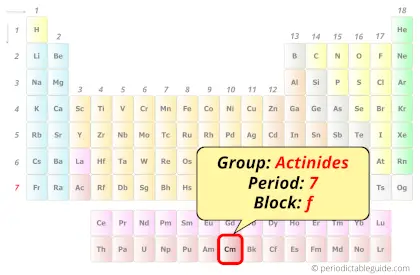 Group: actinides, Period: 7, Block: f |
| Category | 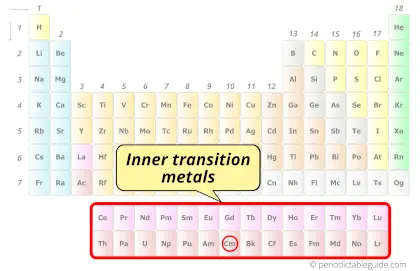 Inner transition metals |
| Atomic number or Protons | 96 |
| Neutrons | 151 |
| Electrons | 96 |
| Symbol | Cm |
| Atomic mass of Curium (most stable isotope) | 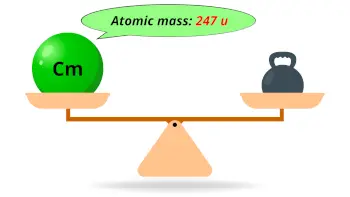 247 u |
| Electrons arrangement or Bohr model | 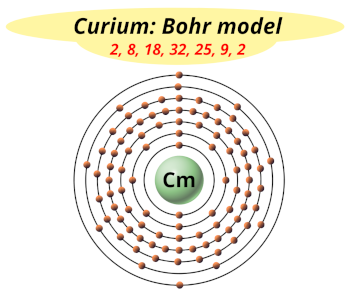 2, 8, 18, 32, 25, 9, 2 |
| Electronic configuration | [Rn] 5f7 6d1 7s2 |
| Atomic radius | 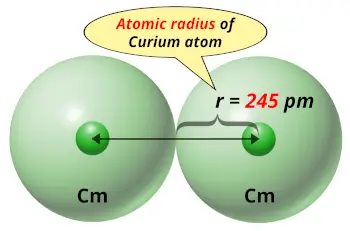 245 picometers (van der Waals radius) |
| 1st Ionization energy | 6.02 eV |
| Electronegativity | 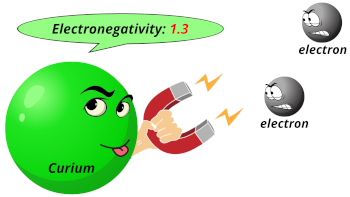 1.3 (Pauling scale) |
| Crystal structure | 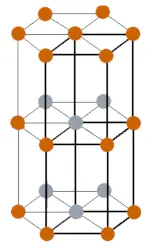 Double hexagonal close packed |
| Melting point | 1613 K or 1340 °C or 2444 °F |
| Boiling point | 3383 K or 3110 °C or 5630 °F |
| Density | 13.51 g/cm3 |
| Main isotopes | 243Cm and 248Cm |
| Who discovered Curium and when? | Glenn T. Seaborg, Albert Ghiorso and Ralph A. James (in 1944) |
| CAS number | 7440-51-9 |
Curium in Periodic table
Curium element is in period 7 and in actinides group of the Periodic table. Curium is the f-block element and it belongs to inner transition metals group.
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba | La* | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| *Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| **Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
←Move to: Americium (Am) element – Periodic Table
→Move to: Berkelium (Bk) element – Periodic Table
Why is Curium in Period 7?
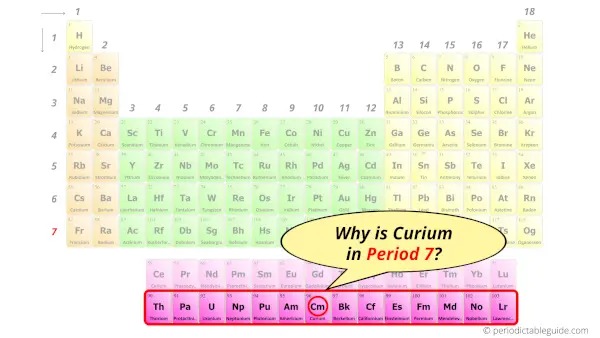
Let me ask you a question.
How many shells does curium have?
It’s 7. Right?
You have already seen the bohr model of curium atom in the above table.
From the Bohr model, it can be found that the number of orbits or shells in curium is 7. Hence, as curium has 7 orbits, it lies in period 7 of the Periodic table.
Why is Curium in f-block?
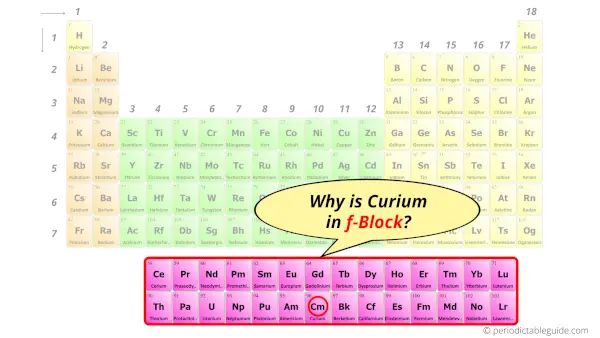
Before knowing this reason, first of all I want to ask you a simple question.
How can you determine the blocks-wise position of elements?
The simple answer: The elements will lie in the s, p, d or f block will completely depend upon the subshell in which the last electron will enter.
For example; the electron configuration of curium is [Rn] 5f7 6d1 7s2.
So the last electron of curium enters the f-subshell or f-orbital.
Hence, curium is the f-block element.
5 Interesting facts about Curium
Interesting facts about curium element are mentioned below.
- The name of the element “Curium” was named after the well known chemists Marie Curie and Pierre Curie.
- Curium was discovered by Glenn T. Seaborg, Albert Ghiorso and Ralph A. James in 1944.
- Curium is prepared synthetically in the laboratory, but it also occurs naturally in trace amounts in uranium containing ores.
- Curium has around 19 isotopes, and all these isotopes are radioactive in nature.
- The most stable isotope of curium is 247Cm, which has a half-life of 15.6 million years.
Properties of Curium
The physical and chemical properties of curium element are mentioned below.
Physical properties of Curium
Physical properties of curium are mentioned below.
- Curium metal is solid in state at room temperature and it has a silvery white metallic luster.
- Curium is a dense metal and its melting point is 1340 °C which is higher than its preceding transuranic elements neptunium, plutonium, and americium.
- At room temperature, the crystal structure of curium is DHCP (double hexagonal close packed) and at higher temperature, its crystal structure changes to face centered cubic.
- The predicted atomic mass of the most stable isotope of curium is 247 u and its density is 13.51 g/cm3.
Chemical properties of Curium
Chemical properties of curium are mentioned below.
- Curium is a chemically reactive metal which oxides in the air when kept open. Due to the oxidation of curium, it forms a thin oxide layer on it.
- Curium is a radioactive element and it is dangerous for the human body as its radiation destroys the red blood cell formation in the bone marrow.
- Curium ions show +3 oxidation state in solutions, and it shows +4 oxidation states in its solid compounds.
Uses of Curium
The uses of curium are mentioned below.
- Due to the radioactive nature of curium element, it is mainly used for research work.
- Curium can be used in radioisotope thermoelectric generators that are used in spacecraft.
- 244Cm is the isotope which was used in X-ray spectrometers to measure the abundance of chemical elements in the rocks and soils on Mars.
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
External resources:
- Curium – Element information, properties and uses | Periodic Table. (n.d.). Curium – Element Information, Properties and Uses | Periodic Table. https://www.rsc.org/periodic-table/element/96/curium
- It’s Elemental – The Element Curium. (n.d.). It’s Elemental – the Element Curium. https://education.jlab.org/itselemental/ele096.html
- P. (n.d.). Curium | Cm (Element) – PubChem. Curium | Cm (Element) – PubChem. https://pubchem.ncbi.nlm.nih.gov/element/Curium
- Curium – Wikipedia. (2008, December 7). Curium – Wikipedia. https://en.wikipedia.org/wiki/Curium
- Curium. (n.d.). Curium. https://www.cs.mcgill.ca/~rwest/wikispeedia/wpcd/wp/c/Curium.htm
- C&EN: IT’S ELEMENTAL: THE PERIODIC TABLE – CURIUM. (n.d.). C&EN: IT’S ELEMENTAL: THE PERIODIC TABLE – CURIUM. https://pubsapp.acs.org/cen/80th/curium.html?
- Periodic Table of Nottingham – University of Nottingham. (n.d.). Periodic Table of Nottingham – University of Nottingham. https://www.nottingham.ac.uk/periodicnottingham/curium
