This is a SUPER easy guide on Oxygen element.
In fact, the table mentioned below is the perfect information box (Which gives you every single detail about the Oxygen element in Periodic table.)
So if you want to know anything about Oxygen element, then this guide is for you.
Let’s finish this very quickly.
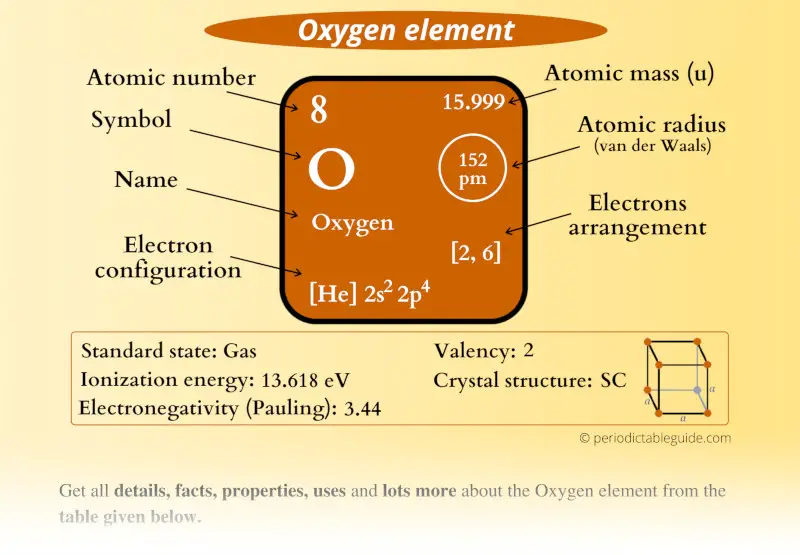
Oxygen Element (O) Information
| Appearance | Colorless gas |
| State (at STP) | Gas |
| Position in Periodic table | 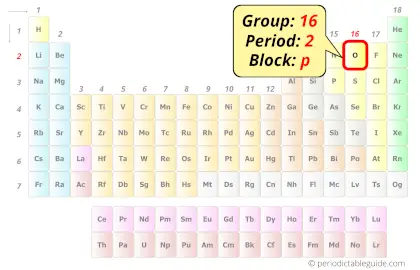 Group: 16, Period: 2, Block: p |
| Category | 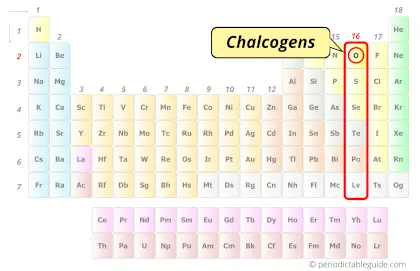 Chalcogens |
| Atomic number or Protons | 8 |
| Neutrons | 8 |
| Electrons | 8 |
| Symbol | O |
| Atomic mass | 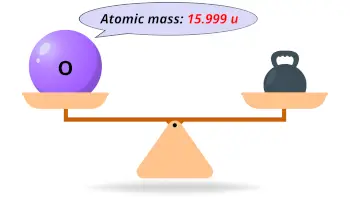 15.999 u |
| Electrons arrangement or Bohr model | 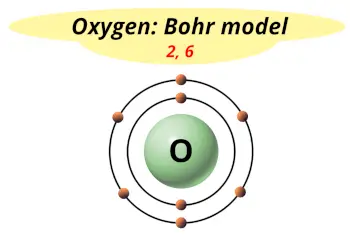 2, 6 |
| Electronic configuration | [He] 2s2 2p4 |
| Atomic radius | 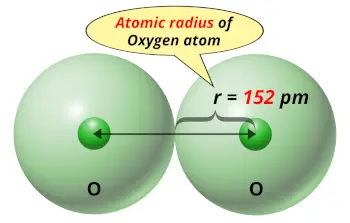 152 picometers (van der Waals radius) |
| Valence electrons | 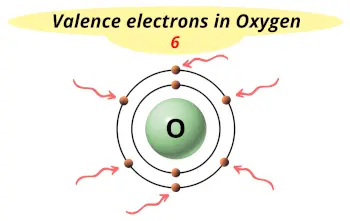 6 |
| 1st Ionization energy | 13.61 eV |
| Electronegativity | 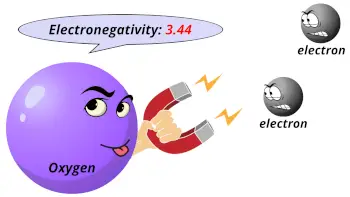 3.44 (Pauling scale) |
| Crystal structure | 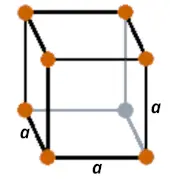 SC (Simple cubic) |
| Melting point of Oxygen (O2) | 54.36 K or -218.79 °C or -361.82 °F |
| Boiling point of Oxygen (O2) | 90.188 K or -182.962 °C or -297.332 °F |
| Density | 1.43 g/L |
| Main isotope | 16O |
| Who discovered Oxygen and when? |  Carl Wilhelm Scheele in 1771 |
| CAS number | 7781-44-7 |
Oxygen in Periodic table
Oxygen element is in group 16 and period 2 of the Periodic table. Oxygen is the p-block element and it belongs to chalcogens group.
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba | La* | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| *Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| **Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
←Move to: Nitrogen (N) element – Periodic Table
→Move to: Fluorine (Fl) element – Periodic Table
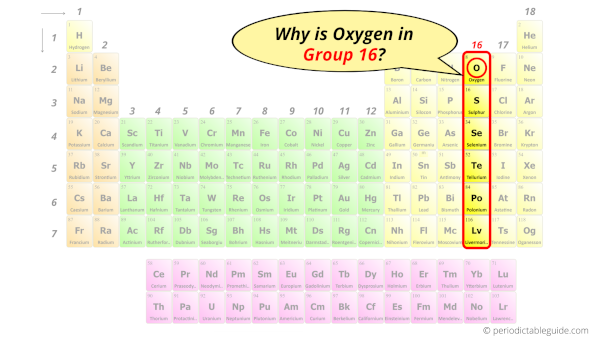
Why is Oxygen in Group 16?
Do you know, how many electrons can be accommodated in the first shell, second shell, third shell, fourth shell, etc…?
Here is the table showing the capacity of orbits to hold electrons.
Number of electrons in shells.
| Orbit / Shell (n) | Maximum no. of electrons this orbit can hold |
| K shell, n = 1 | 2 × 1² = 2 |
| L shell, n = 2 | 2 × 2² = 8 |
| M shell, n = 3 | 2 × 3² = 18 |
| N shell, n = 4 | 2 × 4² = 32 |
Thus,
- 1st shell can hold 2 electrons.
- 2nd shell can hold 8 electrons.
- 3rd shell can hold 18 electrons.
- 4th shell can hold 32 electrons.
Now the atomic number of oxygen (O) is 8.
Hence the oxygen element has electrons arrangement 2, 6.
This electron arrangement indicates that the outermost orbit of Oxygen element (O) has 6 electrons.
Hence, it lies in group 16.
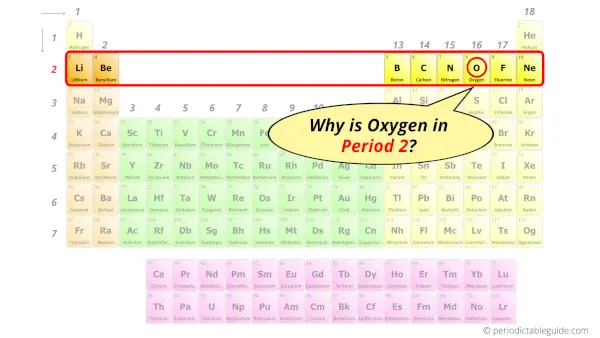
Why is Oxygen in Period 2?
Let me ask you a question.
How many shells does oxygen have?
It’s 2. Right?
You have already seen the bohr model of oxygen atom in the above table.
From the Bohr model, it can be found that the number of orbits or shells in oxygen is 2. Hence, as oxygen has 2 orbits, it lies in period 2 of the Periodic table.
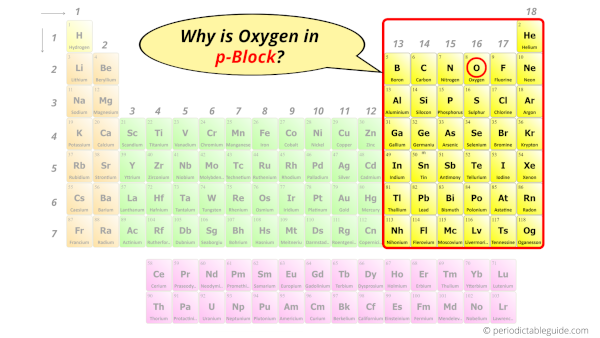
Why is Oxygen in p-block?
Before knowing this reason, first of all I want to ask you a simple question.
How can you determine the blocks-wise position of elements?
The simple answer: The elements will lie in the s, p, d or f block will completely depend upon the subshell in which the last electron will enter.
For example; the electron configuration of oxygen is [He] 2s2 2p4.
So the last electron of oxygen enters the p-subshell or p-orbital.
Hence, oxygen is the p-block element.
10 Interesting facts about Oxygen
Interesting facts about oxygen element are mentioned below.
- Oxygen is colorless gas, but it appears pale-blue in its liquid state.
- All living organisms including plants and animals require oxygen for survival.
- Oxygen normally exists in a divalent molecule (i.e O2). Ozone is another pure form of oxygen and it exists as O3.
- Oxygen gas does not burn itself, but helps in the combustion process. Hence it is also said to be the helper of combustion.
- Oxygen is also present in the human body in a very large proportion. Because the human body is made up of 75% water, which includes oxygen and hydrogen molecules. The number of hydrogen atoms are double than the oxygen atoms, but if we talk about the mass, then oxygen atoms have more mass as compared to hydrogen. Hence ⅔ rd of the human body mass is because of oxygen.
- Oxygen element is the third most abundant element in the universe.
- Oxygen gas is present in the atmosphere (approximately 21%).
- The sea water contains approximately 4.95 ml of dissolved oxygen per litre.
- Plants produce oxygen by photosynthesis, but the majority of oxygen on the earth comes from the sea plants called phytoplankton.
- Oxygen molecules can withstand pressures as high as 19 million times the atmospheric pressure.
Properties of Oxygen
The physical and chemical properties of oxygen element are mentioned below.
Physical properties of Oxygen
Physical properties of oxygen are mentioned below.
- Oxygen is a colourless, odourless and tasteless gas. Oxygen appears pale-blue in color in its liquid state.
- Solubility of oxygen gas in water is more than that of nitrogen element. And this solubility is also temperature dependent.
- Oxygen condenses at 90.188 K temperature and it freezes at 54.36 K temperature.
- Dioxygen (O2) is the most common allotrope of oxygen element which living organisms require for respiration. The other allotrope of oxygen is O3 (ozone) which is found in the upper atmosphere of the earth.
- The physical properties of oxygen and ozone are not the same. Ozone is bluish in color in gaseous as well as liquid state.
Chemical properties of Oxygen
Chemical properties of oxygen are mentioned below.
- At standard temperature and pressure, two oxygen elements combine with each other to form a stable molecule O2 (i.e dioxygen).
- Oxygen element is a reactive nonmetal that forms oxides with most of the other elements.
- Out of all the elements on periodic table, the oxygen element is a second strong oxidizing agent after fluorine element.
- Oxygen does not burn itself, but it helps in combustion. In simple words, it helps other substances to burn. Hence oxygen is also known as a helper of combustion.
- Oxygen also reacts with the metals at room temperature. For example, rusting of metals takes place at room temperature when they react with moisture and oxygen of the air.
Uses of Oxygen
Uses of oxygen are mentioned below.
- In industries, oxygen is used along with other fuel gases. For example oxygen is used with acetylene gas in oxyacetylene flame welding (or gas welding).
- In steel industries, oxygen gas is required to increase the combustion temperatures in blast furnaces.
- Oxygen is used in biological treatment of sewage water plants. This is because the supply of oxygen reduces the formation of hydrogen sulphide and hence the corrosion and odour can be reduced.
- Liquid oxygen is used as an oxidizing agent in liquid fueled rockets, which is required to produce a very high amount of thrust during a takeoff.
- Oxygen is used in many breathing apparatus in hospitals. Oxygen tanks are also used in some underwater work, scuba diving as well as it is also used by mountaineers as the amount of O2 gas decreases at higher altitude.
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
External resources:
- Oxygen – Energy Education. (n.d.). Oxygen – Energy Education. https://energyeducation.ca/encyclopedia/Oxygen
- Oxygen | Center for Science Education. (n.d.). Oxygen | Center for Science Education. https://scied.ucar.edu/learning-zone/air-quality/oxygen
- Oxygen – Element information, properties and uses | Periodic Table. (n.d.). Oxygen – Element Information, Properties and Uses | Periodic Table. https://www.rsc.org/periodic-table/element/8/oxygen
- It’s Elemental – The Element Oxygen. (n.d.). It’s Elemental – the Element Oxygen. https://education.jlab.org/itselemental/ele008.html
