This is a SUPER easy guide on Rubidium element.
In fact, the table mentioned below is the perfect information box (Which gives you every single detail about the Rubidium element in Periodic table.)
So if you want to know anything about Rubidium element, then this guide is for you.
Let’s finish this very quickly.
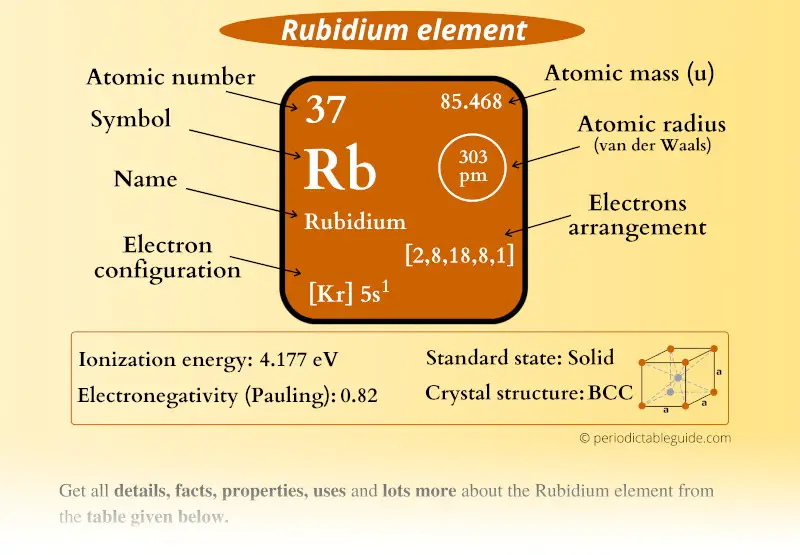
Rubidium Element (Rb) Information
| Appearance |  Grey white |
| State (at STP) | Solid |
| Position in Periodic table | 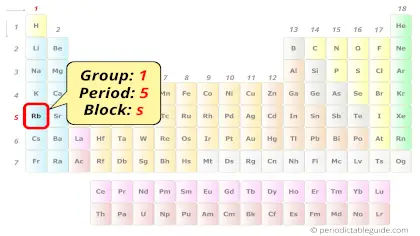 Group: 1, Period: 5, Block: s |
| Category | 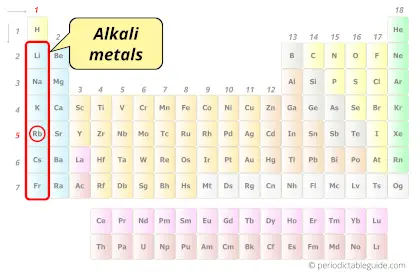 Alkali metals |
| Atomic number or Protons | 37 |
| Neutrons | 48 |
| Electrons | 37 |
| Symbol | Rb |
| Atomic mass | 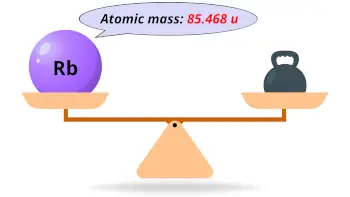 85.468 u |
| Electrons arrangement or Bohr model | 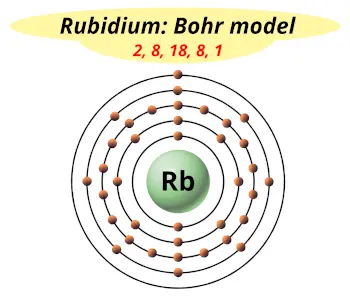 2, 8, 18, 8, 1 |
| Electronic configuration | [Kr] 5s1 |
| Atomic radius | 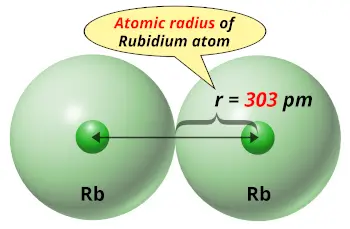 303 picometers (van der Waals radius) |
| Valence electrons | 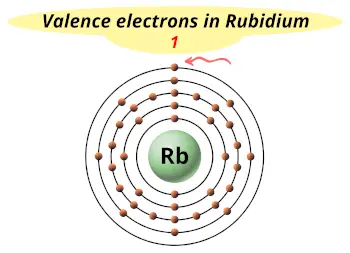 1 |
| 1st Ionization energy | 4.177 eV |
| Electronegativity | 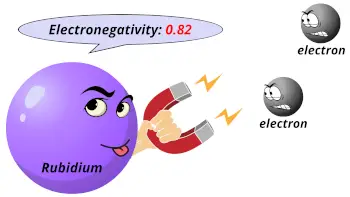 0.82 (Pauling scale) |
| Crystal structure | 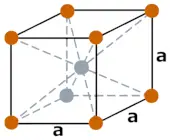 BCC (Body Centered Cubic) |
| Melting point | 312.4 K or 39.3 °C or 102.7 °F |
| Boiling point | 961 K or 688 °C or 1270 °F |
| Density | 1.53 g/cm3 |
| Main isotope | 85Rb, 87Rb |
| Who discovered Rubidium and when? |  Robert Bunsen and Gustav Kirchhoff (in 1861) |
| CAS number | 7440-17-7 |
Rubidium in Periodic table
Rubidium element is in group 1 and period 5 of the Periodic table. Rubidium is the s-block element and it belongs to Alkali metals group.
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba | La* | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| *Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| **Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
←Move to: Krypton (Kr) element – Periodic Table
→Move to: Strontium (Sr) element – Periodic Table
Why is Rubidium in Group 1?
Do you know, how many electrons can be accommodated in the first shell, second shell, third shell, fourth shell, etc…?
Here is the table showing the capacity of orbits to hold electrons.
Number of electrons in shells.
| Orbit / Shell (n) | Maximum no. of electrons this orbit can hold |
| K shell, n = 1 | 2 × 1² = 2 |
| L shell, n = 2 | 2 × 2² = 8 |
| M shell, n = 3 | 2 × 3² = 18 |
| N shell, n = 4 | 2 × 4² = 32 |
Thus,
- 1st shell can hold 2 electrons.
- 2nd shell can hold 8 electrons.
- 3rd shell can hold 18 electrons.
- 4th shell can hold 32 electrons.
Now the atomic number of Rubidium (Rb) is 37.
Hence, the rubidium element has the electrons arrangement 2, 8, 18, 8, 1.
This electron arrangement indicates that the outermost orbit of Rubidium (Rb) has 1 electron.
Hence, it lies in group 1.
Why is Rubidium so reactive?
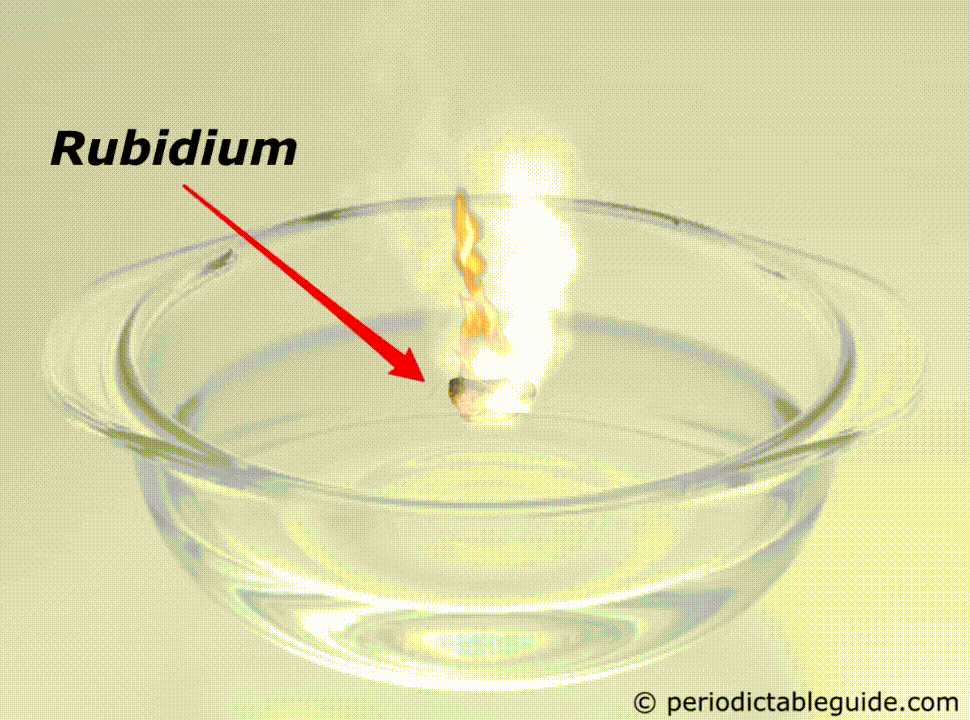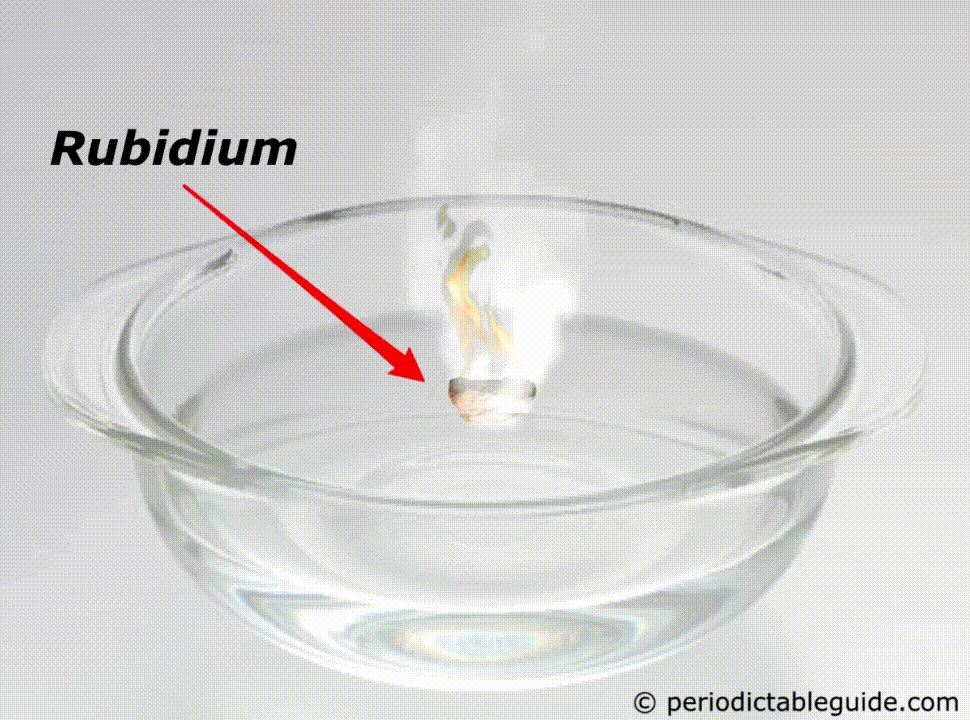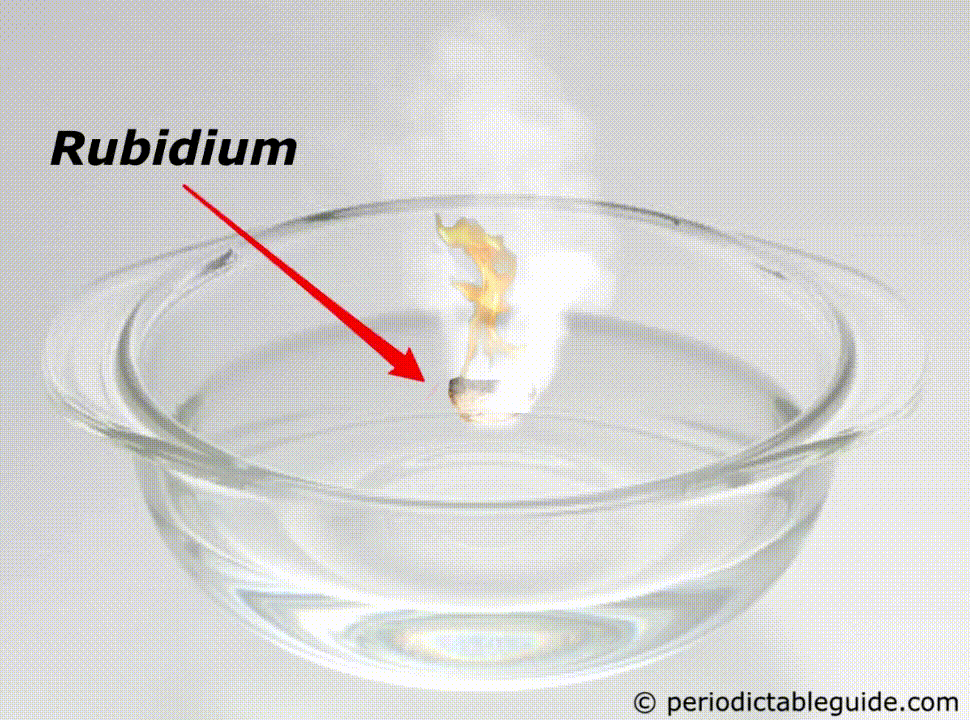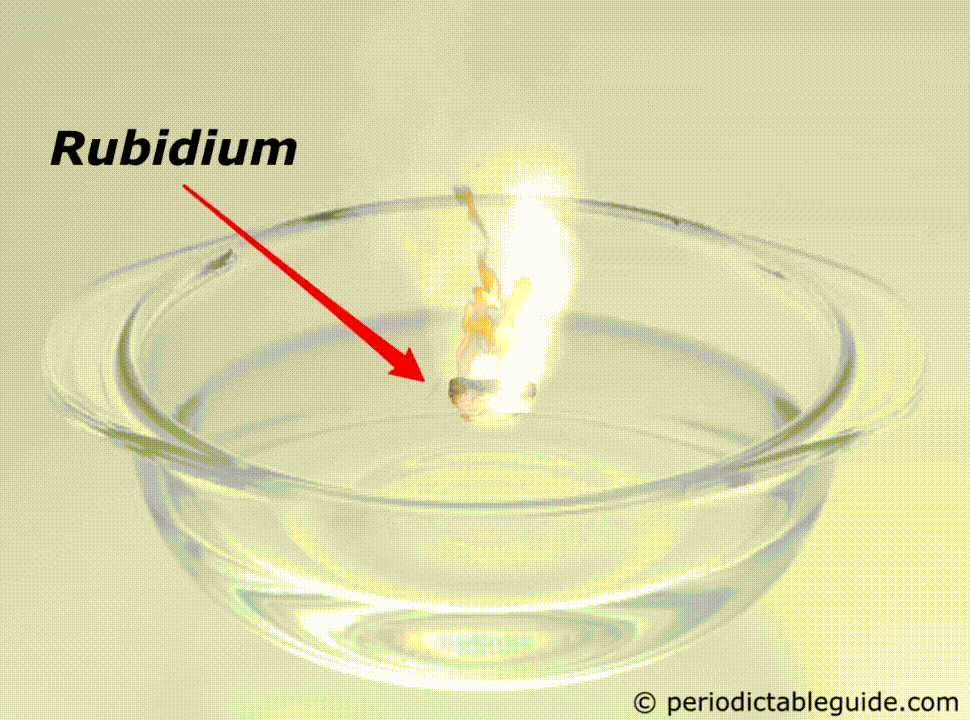
Rubidium is a very reactive metal because of the following reasons.
- Large atomic size
- One valence electron
- Less ionization energy
- Lower boiling point
- Lower melting point
- Less density
- Weak metallic bonds
Explanation:
Rubidium is a group 1 element and it has bigger atomic size compared to the elements of other groups.
Also it possesses only one electron in the outermost orbit.
It has a very less Ionization energy, that means very less energy is required to remove the valence electrons from its orbit.
Also, Rubidium has lower melting point and lower boiling point which indicates that it has weak metallic bonds.
Thus because of all these reasons, Rubidium element can donate or lose its electron very easily.
And hence, Rubidium element is highly reactive.
It is so reactive that if it is kept in the water, it will easily catch fire (See above animation).
Also see: Reaction of Alkali metals with water (Animation + Chemical reactions)
Is Rubidium a Metal, Nonmetal or Metalloid?
Rubidium is a Metal. It’s a grey white colored reactive metal. It is a soft and light metal. It is so reactive that it even catches fire if it comes in contact with water.

But do you know what exactly the metals are?
- Metals are the elements which lose electron/s during a chemical reaction.
In other words, metals are electron donors.
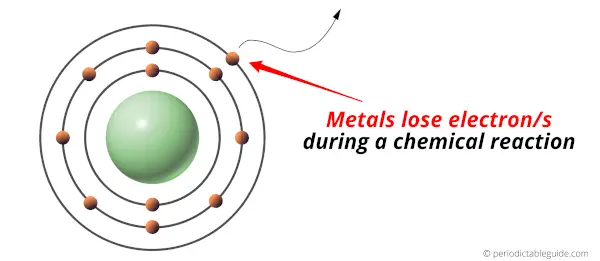
Metals are found on the left side of the Periodic table.
For more clarification, see the location of metals on the Periodic table (Image).
Is Rubidium an Alkali Metal? Why?
Yes, Rubidium is an Alkali Metal.
Reason:
When Rubidium metal reacts with water, it forms alkali (i.e strong base).
Let me explain this to you with a chemical reaction.
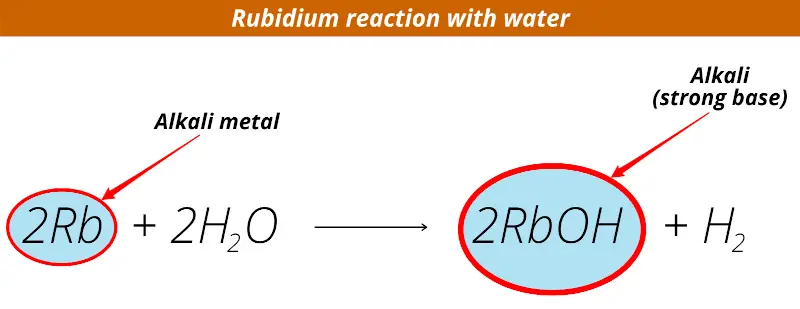
When Rubidium reacts with water, Rubidium hydroxide (RbOH) is obtained.
And this Rubidium hydroxide is alkaline in nature.
So rubidium is known as alkali metal.
In simple words, Rubidium is a metal which forms a strong base (alkalis or alkaline solution) on reacting with water, so it is known as alkali metal.
Also see: Why are Alkaline Earth Metals called so?
Why is Rubidium larger than Xenon?
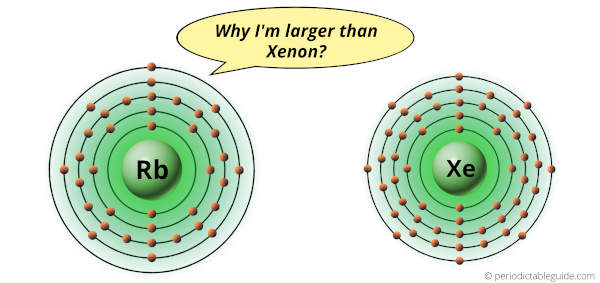
Rubidium and Xenon are the elements in the same period (i.e period 5).
But there is a huge difference in their atomic size.
Van der Waals atomic radius of rubidium is 303 picometers while that of xenon is 216 picometers.
Rubidium atom is bigger in size than the Xenon atom.
Here is the reason behind this.
You might be knowing about the atomic size trend in Periodic table.
According to the atomic size trend, as we move across a period (from left to right in the Periodic table), the atomic size of the elements decreases.
This is because of the increase in attractive force between the positively charged nucleus and negativity charges electrons.
Let me explain this with an example.
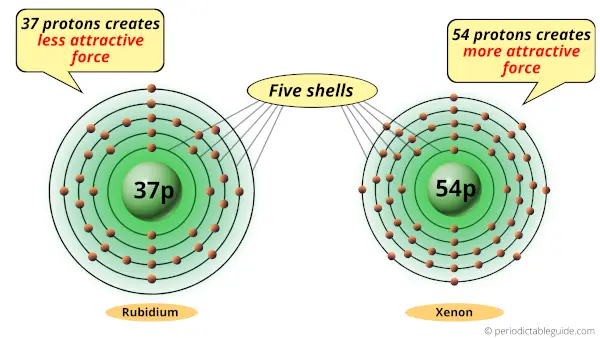
As you can see, Rubidium and Xenon atoms have 5 shells or orbits.
But the difference is the number of protons present in their nucleus.
Rubidium atom has 37 protons while the Xenon atom has 54 protons.
Protons are the positively charged particles which attract the negatively charged electrons.
Now, the xenon atom has 54 protons, so it generates more attractive force compared to that of rubidium.
So if we compare rubidium atom and xenon atom, then they have same number of shells but xenon atom has more positive charge which attracts the electrons towards the nucleus.
Thus, because of this attractive force, the xenon atom shrinks.
Hence rubidium is larger than xenon.
Also see: Periodic table with atomic size labeled on it (Image)
Interesting Facts about Rubidium
Interesting facts about the Rubidium element are mentioned below.
- Rubidium is not found in a pure form due to its highly reactive nature. It is always found in compound forms with other elements. Many times rubidium is found with cesium and other Alkali metals. In such cases, it becomes very difficult to separate them, as all the alkali metals have similar characteristics.
- Density of rubidium is 1.5 times that of water.
- Flame test of Rubidium gives red-violet color. So, the red-violet color in fireworks can be obtained by using the Rubidium element.
- Rubidium is the second most electropositive element. (source)
- Rubidium is 23rd most abundant element in the earth’s crust.
- Melting point of Rubidium is just slightly more than human body temperature. (Our average body temperature is 37 °C, and the melting point of Rubidium is 39.3 °C).
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
External resources:
- It’s Elemental – The Element Rubidium. (n.d.). It’s Elemental – the Element Rubidium. https://education.jlab.org/itselemental/ele037.html
- Georgescu, I. (2015, November 20). Rubidium round-the-clock. Nature Chemistry, 7(12), 1034–1034. https://doi.org/10.1038/nchem.2407
- Simmons, E. C. (n.d.). Rubidium: Element and geochemistry. Encyclopedia of Earth Science, 555–556. https://doi.org/10.1007/1-4020-4496-8_278
- Rubidium – Element information, properties and uses | Periodic Table. (n.d.). Rubidium – Element Information, Properties and Uses | Periodic Table. https://www.rsc.org/periodic-table/element/37/rubidium
- P. (n.d.). Rubidium | Rb (Element) – PubChem. Rubidium | Rb (Element) – PubChem. https://pubchem.ncbi.nlm.nih.gov/element/Rubidium
