This is a SUPER easy guide on Selenium element.
In fact, the table mentioned below is the perfect information box (Which gives you every single detail about the Selenium element in Periodic table.)
So if you want to know anything about Selenium element, then this guide is for you.
Let’s finish this very quickly.
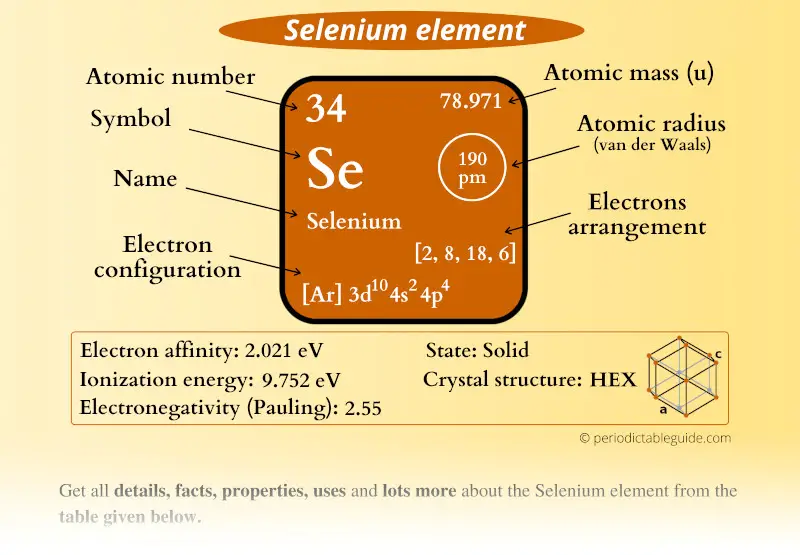
Selenium Element (Se) Information
| Appearance |  Hexagonal selenium is: Metallic-grey, Monoclinic selenium is: Deep red |
| State (at STP) | Solid |
| Position in Periodic table | 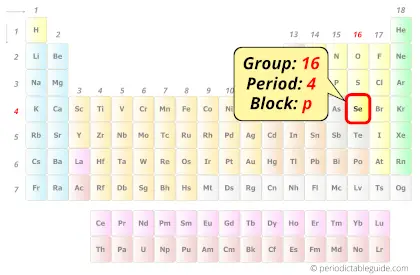 Group: 16, Period: 4, Block: p |
| Category | 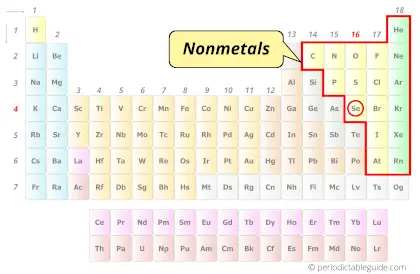 Nonmetals |
| Atomic number or Protons | 34 |
| Neutrons | 45 |
| Electrons | 34 |
| Symbol | Se |
| Atomic mass | 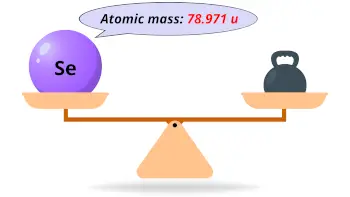 78.971 u |
| Electrons arrangement or Bohr model | 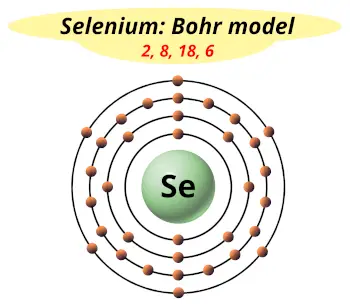 2, 8, 18, 6 |
| Electronic configuration | [Ar] 3d10 4s2 4p4 |
| Atomic radius | 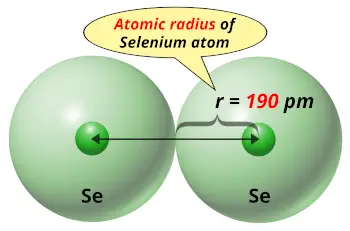 190 picometers (van der Waals radius) |
| Valence electrons | 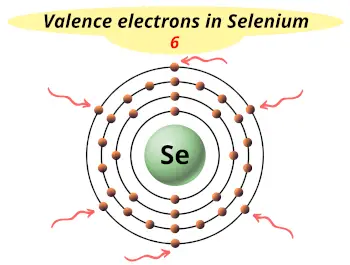 6 |
| 1st Ionization energy | 9.752 eV |
| Electronegativity | 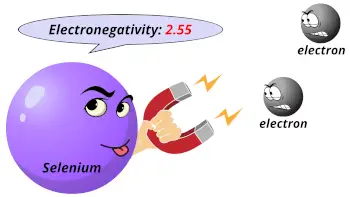 2.55 (Pauling scale) |
| Crystal structure | 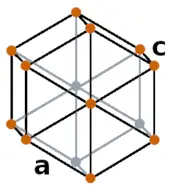 HEX (Hexagonal) |
| Melting point | 494 K or 221 °C or 430 °F |
| Boiling point | 958 K or 685 °C or 1265 °F |
| Density | 4.82 g/cm3 |
| Main isotope | 80Se |
| Who discovered Selenium and when? |  Jöns Jakob Berzelius and Johann Gottlieb Gahn (In 1817) |
| CAS number | 7482-49-2 |
Selenium in Periodic table
Selenium element is in group 16 and period 4 of the Periodic table. Selenium is the p-block element and it belongs to the Nonmetals – oxygen group (aka chalcogens).
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba | La* | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| *Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| **Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
←Move to: Arsenic (As) element – Periodic Table
→Move to: Bromine (Br) element – Periodic Table
What type of Element is Selenium? (Metal, Nonmetal or Metalloid?)
Selenium is a Nonmetal.
It is also true that selenium shows some properties of Metalloids.
But according to the properties of Metalloids, selenium shows only 24% properties of Metalloids.
And most of its properties are similar to that of nonmetals.
Let me tell you what properties selenium shows?
Few Metallic properties:
The Hexagonal selenium which is grey in color shows a lustrous metallic appearance.
Grey selenium is shiny metallic. It also has good electrical conductivity as compared to that of monoclinic selenium (which is deep red in color).
(Note: Hexagonal selenium and monoclinic selenium are the two different allotropes of selenium)
Also, selenium can be drawn into thin threads when it is molten and viscous.
More Nonmetallic properties:
Selenium is brittle in nature, which shows it’s Nonmetallic characteristics.
If we see the electric conductivity of highly purified selenium, then it is very less (and it is similar to that of bromine).
Selenium shows a higher electronegativity like that of nonmetals.
Also in most of the chemical reactions, selenium gains electrons to become negatively charged ions (Se2-). That means its chemical properties are similar to that of nonmetals.
Thus most of the chemical properties of selenium are the same as that of nonmetals.
Hence, Selenium is classified as a Nonmetals on the periodic table.
Also see: Periodic table with Metals, Nonmetals and Metalloids (Labeled Image).
Why is Selenium named after the Moon?
In 1817, Jöns Jakob Berzelius suggested the name “Selenium” for the newly discovered element.
But the question is;
Why did he suggest this name and from where this name comes from?
Swedish chemists Jöns Jakob Berzelius and Johann Gottlieb Gahn were studying some chemicals for preparation of sulphuric acid.
During this study, they found some impurity and they thought that it would be tellurium.
Later on they realized that they were wrong.
It was not tellurium, but they found a new element which was somewhat similar to tellurium.
But, tellurium was already discovered 30 years before the discovery of this new element.
Jöns Jakob Berzelius suggested the name “selenium” for this newly discovered element.
This name came from the Greek word “Selene”, which means “moon”.
The earlier discovered element (i.e tellurium) was named after the Latin word “Tellus”, which means “earth”.
The properties of the newly discovered element (selenium) were similar to that of tellurium.
Just as the earth and moon go together, so do selenium and tellurium.
Hence in 1817, Jöns Jakob Berzelius suggested the name of a new element as Selenium based on the Greek work “Selene”, meaning “moon”.
Why is Selenium in Group 16 and Period 4 of the Periodic table?
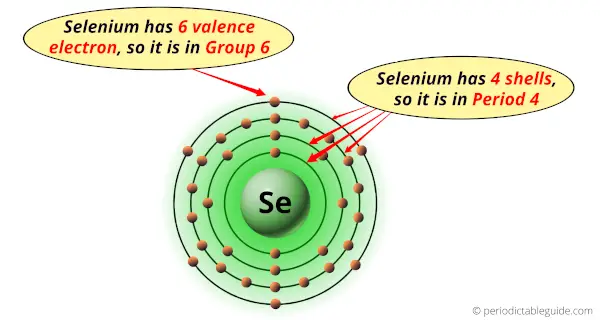
Selenium is in group 16 because it has 6 valence electrons.
Selenium is in period 4 because it has 4 shells or orbits.
Now I will explain to you the perfect reason why selenium is in group 16, why it is in period 4 and also why it is in p-block?
Let’s see the reasons one by one.
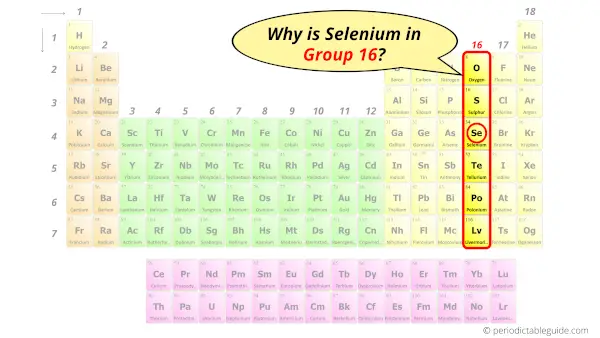
Why is Selenium in Group 16?
Do you know, how many electrons can be accommodated in the first shell, second shell, third shell, fourth shell, etc…?
Here is the table showing the capacity of orbits to hold electrons.
Number of electrons in shells.
| Orbit / Shell (n) | Maximum no. of electrons this orbit can hold |
| K shell, n = 1 | 2 × 1² = 2 |
| L shell, n = 2 | 2 × 2² = 8 |
| M shell, n = 3 | 2 × 3² = 18 |
| N shell, n = 4 | 2 × 4² = 32 |
Thus,
- 1st shell can hold 2 electrons.
- 2nd shell can hold 8 electrons.
- 3rd shell can hold 18 electrons.
- 4th shell can hold 32 electrons.
Now the atomic number of Selenium (Se) is 34.
Hence, selenium element has the electrons arrangement 2, 8, 18, 6.
This electron arrangement indicates that the outermost orbit of selenium (Se) has 6 electrons.
Hence, it lies in group 16.
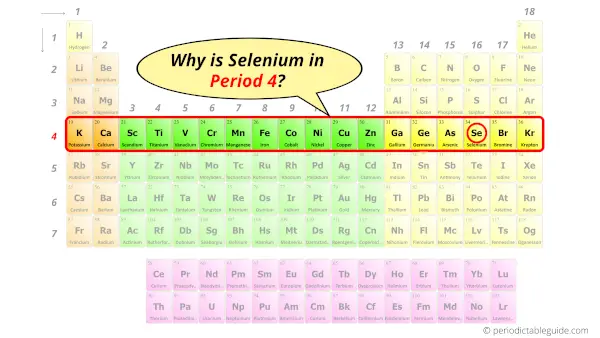
Why is Selenium in Period 4?
Let me ask you a question.
How many shells does selenium have?
It’s 4. Right?
You have already seen this in the bohr model of selenium element in above table.
From the Bohr model, it can be found that the number of orbits or shells in selenium atom is 4.
Hence, as selenium has 4 orbits, it lies in period 4 of the Periodic table.
Also see: Periodic table with electrons per shell (Image)
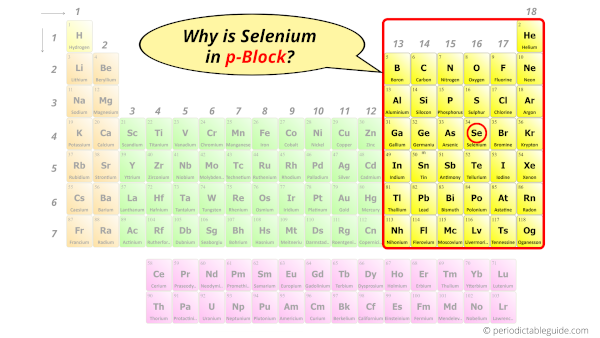
Why is Selenium in p-block?
Before knowing this reason, first of all a simple question to you.
How can you determine the blocks wise position of elements?
The simple answer: The elements will lie in the s, p, d or f block will completely depend upon the subshell in which the last electron will enter.
For example;
The electron configuration of Selenium is [Ar] 3d10 4s2 4p4.
So the last electron of selenium enters the p-subshell or p-orbital.
Hence, selenium is the p-block element.
Interesting Facts about Selenium
Interesting facts about selenium element are mentioned below.
- Jöns Jakob Berzelius suggested the name of a new element as Selenium based on the Greek work “Selene”, meaning “moon”.
- Selene is a Nonmetal and it has different colors and crystal structure (which most of the nonmetals have). For example, Hexagonal selenium has metallic grey color, while monoclinic selenium has deep red color.
- 2000 tons of selenium element is extracted every year worldwide.
- Alexander Graham Bell used selenium in 1879 to make photophone.
- Most of the selenium is produced in Germany, while most of the selenium is consumed in China for industrial purpose.
- Around 50% of Selenium produced is used in the manufacturing of glass.
Free Gift for you: Interactive Periodic Table
Let me tell you how this Interactive Periodic Table will help you in your studies.
1). You can effortlessly find every single detail about the elements from this single Interactive Periodic table.
2). You will get the detailed information about the periodic table which will convert a newbie into pro.
3). You will also get the HD images of the Periodic table (for FREE).
Checkout Interactive Periodic table and download it’s high resolution image now (It’s FREE)
External resources:
- Selenium – Wikipedia. (2022, February 1). Selenium – Wikipedia. https://en.wikipedia.org/wiki/Selenium
- Selenium – Element information, properties and uses | Periodic Table. (n.d.). Selenium – Element Information, Properties and Uses | Periodic Table. https://www.rsc.org/periodic-table/element/34/selenium
- P. (n.d.). Selenium. Selenium | Se – PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/6326970
- It’s Elemental – The Element Selenium. (n.d.). It’s Elemental – the Element Selenium. https://education.jlab.org/itselemental/ele034.html
- P. (n.d.). Selenium | Se (Element) – PubChem. Selenium | Se (Element) – PubChem. https://pubchem.ncbi.nlm.nih.gov/element/Selenium