Electronegativity chart of all elements is mentioned below.
(Note: Electronegativity has no unit. Linus Pauling was a scientist who designed a scale of electronegative that ranks the elements with respect to each other. And this scale is known as Pauling electronegativity scale.)
The Pauling electronegativity values of the element ranges from the most electronegative element (Fluorine having electronegativity = 3.98) to least electronegative element (Francium having electronegativity = 0.7)
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
External links:
Electronegativity of elements

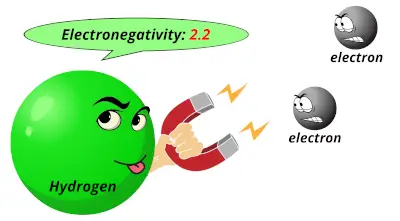
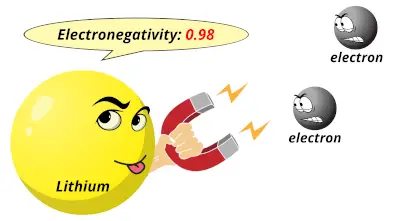 0.98
0.98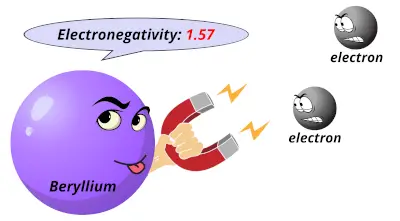 1.57
1.57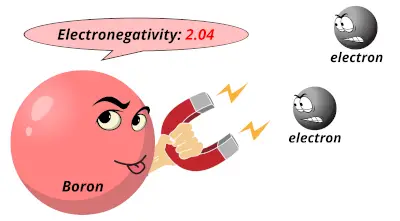 2.04
2.04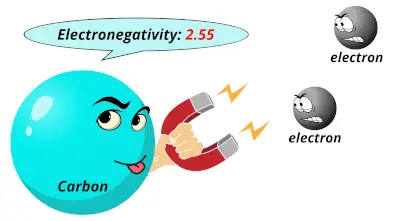 2.55
2.55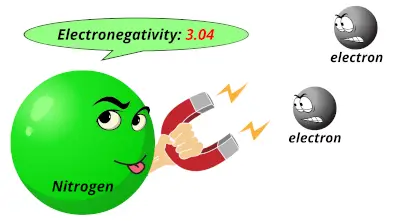 3.04
3.04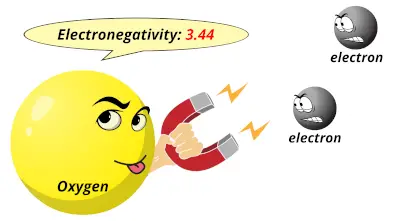 3.44
3.44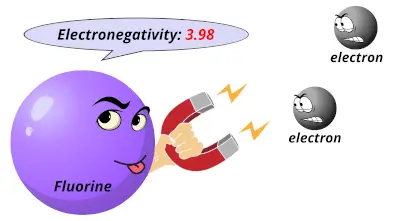 3.98
3.98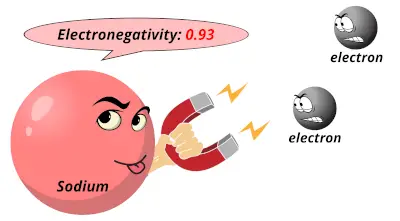 0.93
0.93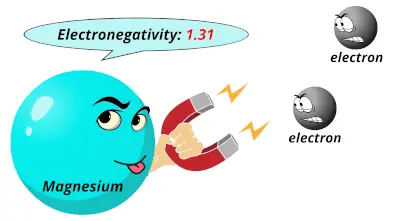 1.31
1.31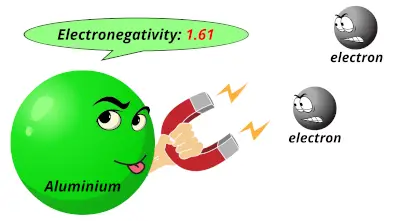 1.61
1.61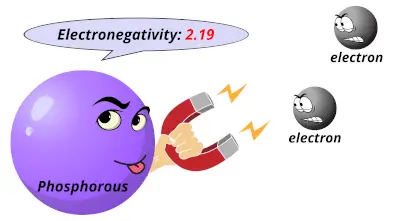 2.19
2.19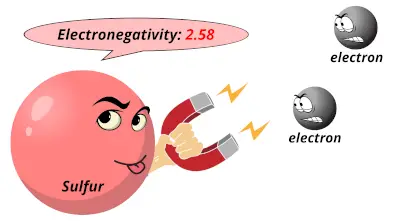 2.58
2.58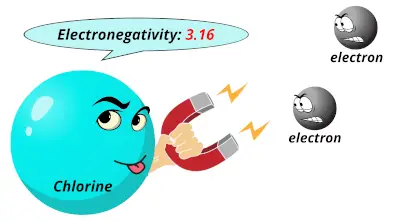 3.16
3.16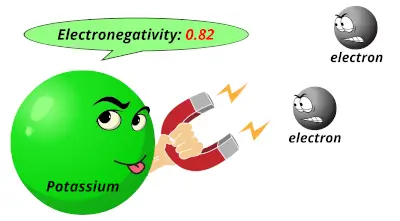 0.82
0.82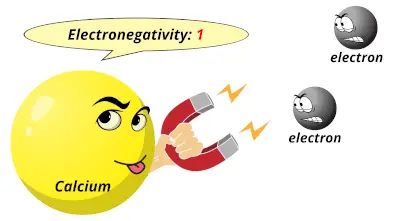 1
1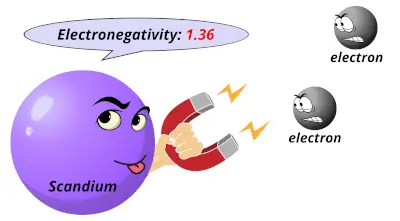 1.36
1.36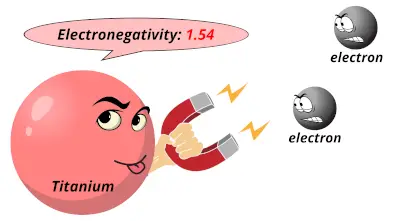 1.54
1.54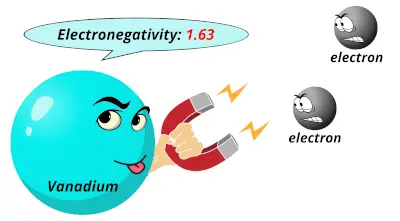 1.63
1.63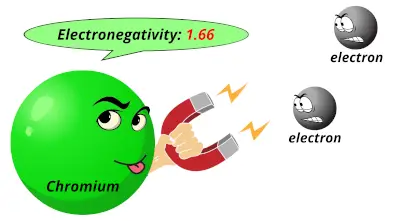 1.66
1.66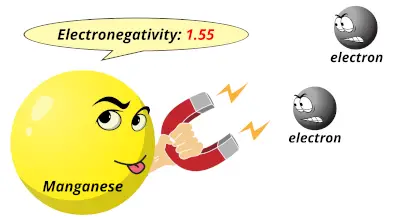 1.55
1.55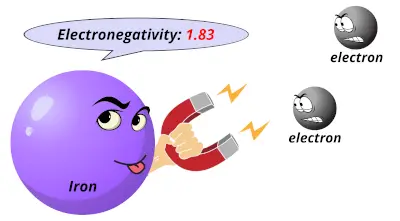 1.83
1.83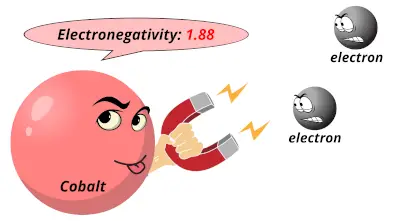 1.88
1.88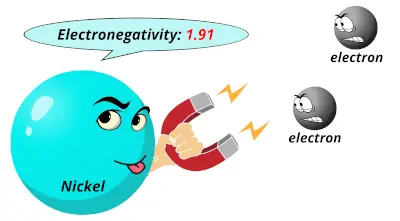 1.91
1.91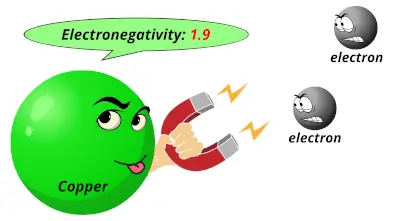 1.9
1.9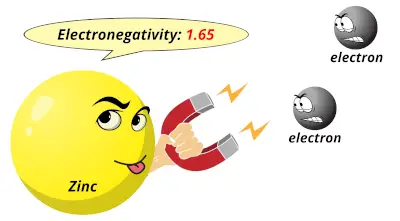 1.65
1.65