This is a SUPER easy guide on Thallium element.
In fact, the table mentioned below is the perfect information box (Which gives you every single detail about the Thallium element in Periodic table.)
So if you want to know anything about Thallium element, then this guide is for you.
Let’s finish this very quickly.
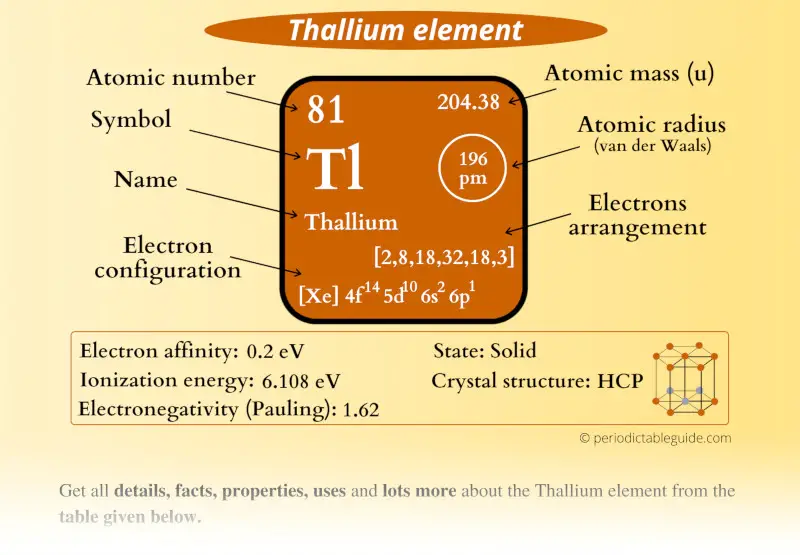
Thallium Element (Tl) Information
| Appearance |  Silvery white metallic |
| State (at STP) | Solid |
| Position in Periodic table | 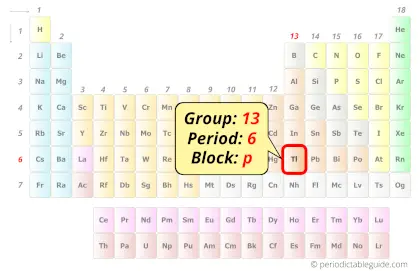 Group: 13, Period: 6, Block: p |
| Category | 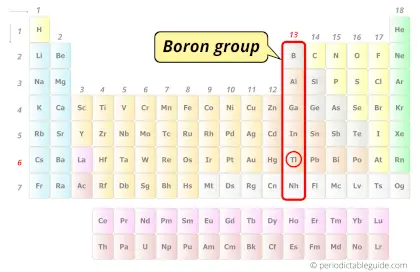 Boron group |
| Atomic number or Protons | 81 |
| Neutrons | 123 |
| Electrons | 81 |
| Symbol | Tl |
| Atomic mass | 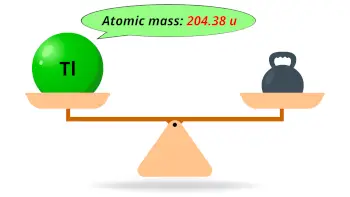 204.38 u |
| Electrons arrangement or Bohr model | 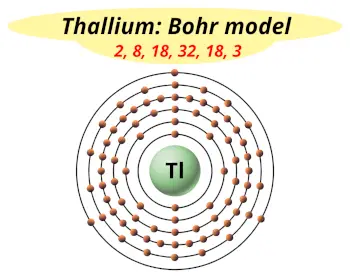 2, 8, 18, 32, 18, 3 |
| Electronic configuration | [Xe] 4f14 5d10 6s2 6p1 |
| Atomic radius | 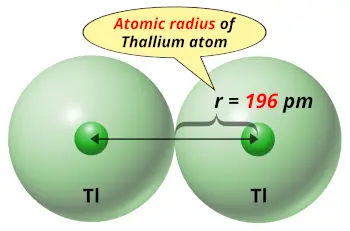 196 picometers (van der Waals radius) |
| Valence electrons | 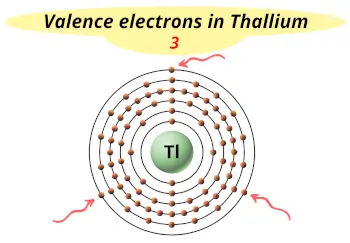 3 |
| 1st Ionization energy | 6.108 eV |
| Electronegativity | 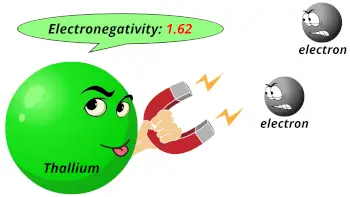 1.62 (Pauling scale) |
| Crystal structure | 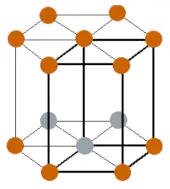 HCP (hexagonal close packed) |
| Melting point | 577 K or 304 °C or 579 °F |
| Boiling point | 1746 K or 1473 °C or 2683 °F |
| Density | 11.85 g/cm3 |
| Main isotope | 205Tl |
| Who discovered Thallium and when? |  William Crookes (in 1861) |
| CAS number | 7440-28-0 |
Thallium in Periodic table
Thallium element is in group 13 and period 6 of the Periodic table. Thallium is the p-block element and it belongs to boron group.
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba | La* | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| *Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| **Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
←Move to: Mercury (Hg) element – Periodic Table
→Move to: Lead (Pb) element – Periodic Table
Why is Thallium in Group 13?
Do you know, how many electrons can be accommodated in the first shell, second shell, third shell, fourth shell, etc…?
Here is the table showing the capacity of orbits to hold electrons.
Number of electrons in shells.
| Orbit / Shell (n) | Maximum no. of electrons this orbit can hold (2 × n2) |
| K shell, n = 1 | 2 × 1² = 2 |
| L shell, n = 2 | 2 × 2² = 8 |
| M shell, n = 3 | 2 × 3² = 18 |
| N shell, n = 4 | 2 × 4² = 32 |
| . . . | . . . |
Thus,
- 1st shell can hold 2 electrons.
- 2nd shell can hold 8 electrons.
- 3rd shell can hold 18 electrons.
- 4th shell can hold 32 electrons.
Now the atomic number of Thallium (Tl) is 81.
Hence the thallium element has electrons arrangement 2, 8, 18, 32, 18, 3.
This electron arrangement indicates that the outermost orbit of thallium element (Tl) has 3 electrons.
Hence, it lies in group 13.
Why is Thallium in Period 6?
Let me ask you a question.
How many shells does thallium have?
It’s 6. Right?
You have already seen the bohr model of thallium atom in the above table.
From the Bohr model, it can be found that the number of orbits or shells in thallium is 6. Hence, as thallium has 6 orbits, it lies in period 6 of the Periodic table.
Why is Thallium in p-block?
Before knowing this reason, first of all I want to ask you a simple question.
How can you determine the blocks-wise position of elements?
The simple answer: The elements will lie in the s, p, d or f block will completely depend upon the subshell in which the last electron will enter.
For example; the electron configuration of thallium is [Xe] 4f14 5d10 6s2 6p1.
So the last electron of thallium enters the p-subshell or p-orbital.
Hence, thallium is the p-block element.
5 Interesting facts about Thallium
Interesting facts about thallium element are mentioned below.
- The name thallium was derived from the Greek word “thallos”, which means green shoot.
- The concentration of thallium in the earth’s crust is around 850 parts per billion by weight.
- Thallium element was discovered by William Crookes in 1861.
- Out of the total production of thallium, around 70% of thallium goes to electronics industries.
- As thallium rapidly oxidizes in the air, it is required to be stored in mineral oil.
Properties of Thallium
The physical and chemical properties of thallium element are mentioned below.
Physical properties of Thallium
Physical properties of thallium are mentioned below.
- Thallium is a solid metal having a silvery white metallic surface.
- Thallium is a soft metal that can be cut even with a kitchen knife.
- There are many isotopes of thallium, out of which the most abundant isotope is 205Tl. It has an abundance of approximately 70.5%.
- The crystal structure of thallium is HCP (i.e hexagonal close packed).
- The atomic mass of thallium is 204.38 u and its density is 11.85 g/cm3.
- The melting point and boiling point of thallium are 304 °C and 1473 °C respectively.
Chemical properties of Thallium
Chemical properties of thallium are mentioned below.
- Thallium is reactive metal and it tarnishes easily when kept open in the air.
- When thallium is present in its compounds, it exists in its monovalent state (i.e Tl+).
- Thallium reacts with water to form thallium hydroxide, which is poisonous.
- The first ionization energy of thallium is 6.108 eV.
- The electronegativity of thallium is 1.62 on the pauling scale.
Uses of Thallium
Uses of thallium are mentioned below.
- Thallium oxide (which is a compound of thallium) is used to manufacture glasses with higher refractive index.
- Thallium metal is also used in the equipment that is used to detect gamma radiation.
- When thallium sulfide is exposed to infrared light, its electrical conductivity increases. Because of this reason, it is used in photocells.
- In electronics industries, thallium is used in semiconductor material for selenium rectifiers. [1]
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
External resources:
- Thallium – Element information, properties and uses | Periodic Table. (n.d.). Thallium – Element Information, Properties and Uses | Periodic Table. https://www.rsc.org/periodic-table/element/81/thallium
- Thallium – Wikipedia. (2008, February 2). Thallium – Wikipedia. https://en.wikipedia.org/wiki/Thallium
- P. (n.d.). Thallium | Tl (Element) – PubChem. Thallium | Tl (Element) – PubChem. https://pubchem.ncbi.nlm.nih.gov/element/Thallium
- It’s Elemental – The Element Thallium. (n.d.). It’s Elemental – the Element Thallium. https://education.jlab.org/itselemental/ele081.html
- Thallium Statistics and Information | U.S. Geological Survey. (n.d.). Thallium Statistics and Information | U.S. Geological Survey. https://www.usgs.gov/centers/national-minerals-information-center/thallium-statistics-and-information
- C&EN: IT’S ELEMENTAL: THE PERIODIC TABLE – THALLIUM. (n.d.). C&EN: IT’S ELEMENTAL: THE PERIODIC TABLE – THALLIUM. https://pubsapp.acs.org/cen/80th/print/thalliumprint.html?
