This is a SUPER easy guide on Europium element.
In fact, the table mentioned below is the perfect information box (Which gives you every single detail about the Europium element in Periodic table.)
So if you want to know anything about Europium element, then this guide is for you.
Let’s dive right into it!
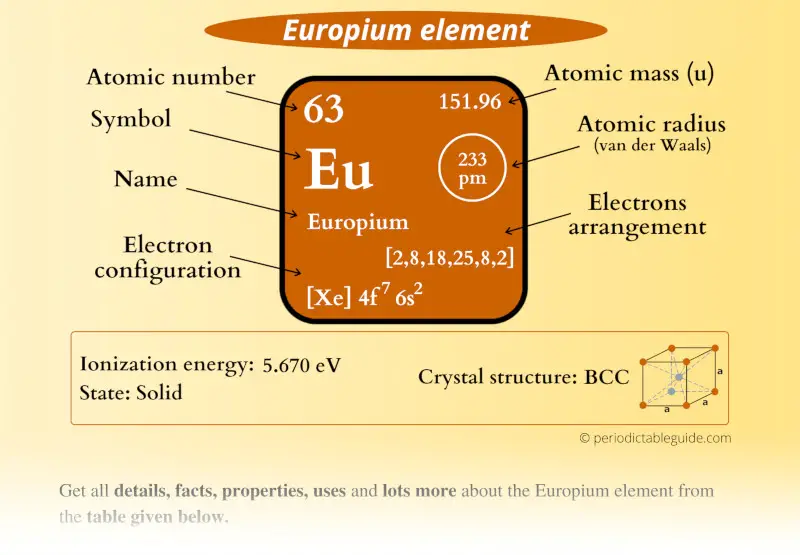
Europium Element (Eu) Information
| Appearance |  Silvery white metallic with pale yellow tint |
| State (at STP) | Solid |
| Position in Periodic table | 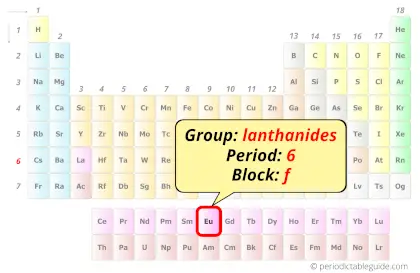 Group: lanthanides, Period: 6, Block: f |
| Category | 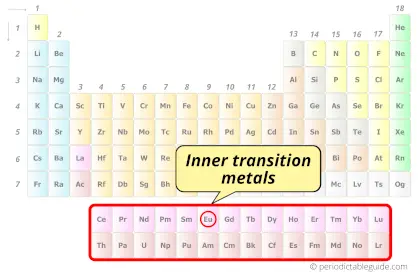 Inner transition metals |
| Atomic number or Protons | 63 |
| Neutrons | 89 |
| Electrons | 63 |
| Symbol | Eu |
| Atomic mass | 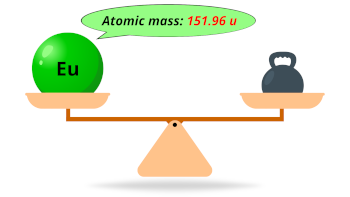 151.96 u |
| Electrons arrangement or Bohr model | 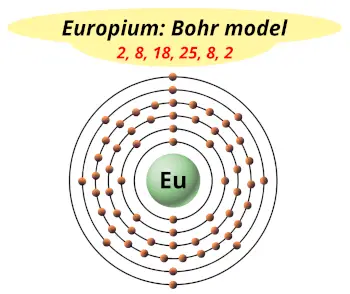 2, 8, 18, 25, 8, 2 |
| Electronic configuration | [Xe] 4f7 6s2 |
| Atomic radius | 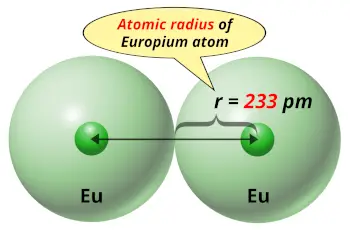 233 picometers (van der Waals radius) |
| 1st Ionization energy | 5.670 eV |
| Crystal structure | 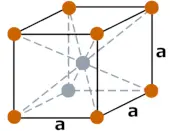 BCC (Body centered cubic) |
| Melting point | 1099 K or 826 °C or 1519 °F |
| Boiling point | 1802 K or 1529 °C or 2784 °F |
| Density | 5.25 g/cm3 |
| Main isotope | 153Eu |
| Who discovered Europium and when? |  Eugene-Anatole Demarcay in 1896 |
| CAS number | 7440-53-1 |
Europium in Periodic table
Europium element is in period 6 and in lanthanide group of the Periodic table. Europium is the f-block element and it belongs to inner transition metals group.
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba | La* | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| *Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| **Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
←Move to: Samarium (Sm) element – Periodic Table
→Move to: Gadolinium (Gd) element – Periodic Table
Why is Europium in Period 6?
Let me ask you a question.
How many shells does europium have?
It’s 6. Right?
You have already seen the bohr model of europium atom in the above table.
From the Bohr model, it can be found that the number of orbits or shells in europium is 6. Hence, as europium has 6 orbits, it lies in period 6 of the Periodic table.
Why is Europium in f-block?
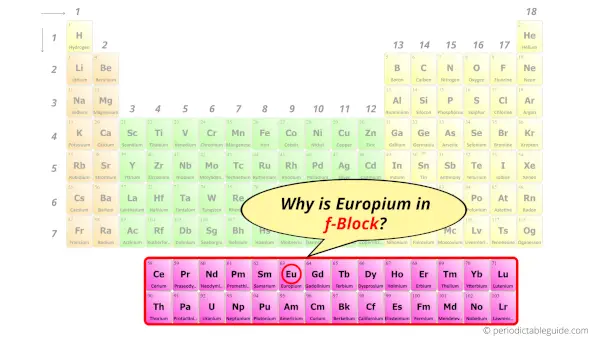
Before knowing this reason, first of all I want to ask you a simple question.
How can you determine the blocks-wise position of elements?
The simple answer: The elements will lie in the s, p, d or f block will completely depend upon the subshell in which the last electron will enter.
For example; the electron configuration of europium is [Xe] 6s2 4f7.
So the last electron of europium enters the f-subshell or f-orbital.
Hence, europium is the f-block element.
5 Interesting facts about Europium
Interesting facts about europium element are mentioned below.
- The name europium came from the continent “Europe”.
- Europium was discovered by Eugene-Anatole Demarcay in 1896.
- Bastnasite and monazite are the ores present in the earth’s crust, from which europium is mostly obtained.
- The proportion of europium present in the earth’s crust is approximately 1.8 parts per million by weight.
- Out of all the rare earth metals present on the periodic table, europium is the most reactive.
Properties of Europium
The physical and chemical properties of europium element are mentioned below.
Physical properties of Europium
Physical properties of europium are mentioned below.
- Europium is a soft and ductile metal having a silvery white appearance with a pale yellow tint.
- The melting point and boiling point of europium element are 826 °C and 1529 °C respectively.
- The atomic mass of europium is 151.96 u and its density is 5.25 g/cm3.
- Europium has a BCC (Body centered cubic) crystal structure.
- Europium has many isotopes, but out of those isotopes the most abundant isotope is 153Eu (having approximately 52% abundance).
Chemical properties of Europium
Chemical properties of europium are mentioned below.
- Europium is a chemically reactive metal and hence it is always present in a compound form in the earth’s crust.
- Like most of the other rare earth metals, the europium element also ignites at the temperature above 150 °C.
- When europium is kept open in the air, it reacts with the oxygen of the air and gets oxidized.
- The reaction of Europium with water is also quick and vigorous. Europium reacts with water to form europium oxide and it liberates hydrogen gas during this reaction.
- The electronic configuration of europium is [Xe] 6s2 4f7, which indicates that the last electron enters the f-orbital. Hence europium is a f-block element.
Uses of Europium
Uses of europium are mentioned below.
- The europium oxide which contains europium is used in screens of TV as well as computers.
- The isotopes of europium are also used in nuclear power plants as a neutron absorber.
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
External resources:
- Europium – Element information, properties and uses | Periodic Table. (n.d.). Europium – Element Information, Properties and Uses | Periodic Table. https://www.rsc.org/periodic-table/element/63/europium
- Europium – Wikipedia. (2009, August 8). Europium – Wikipedia. https://en.wikipedia.org/wiki/Europium
- P. (n.d.). Europium | Eu (Element) – PubChem. Europium | Eu (Element) – PubChem. https://pubchem.ncbi.nlm.nih.gov/element/Europium
- It’s Elemental – The Element Europium. (n.d.). It’s Elemental – the Element Europium. https://education.jlab.org/itselemental/ele063.html
- Atomic Data for Europium (Eu). (n.d.). Atomic Data for Europium (Eu). https://physics.nist.gov/PhysRefData/Handbook/Tables/europiumtable1.htm
