This is a SUPER easy guide on Zinc element.
In fact, the table mentioned below is the perfect information box (Which gives you every single detail about the Zinc element in Periodic table.)
So if you want to know anything about Zinc element, then this guide is for you.
Let’s dive right into it!
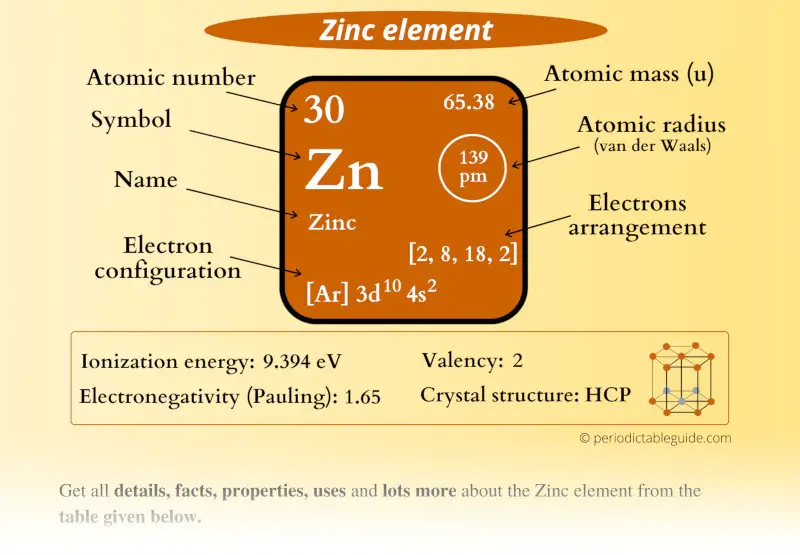
Zinc Element (Zn) Information
| Appearance |  Silvery grey |
| State (at STP) | Solid |
| Position in Periodic table | 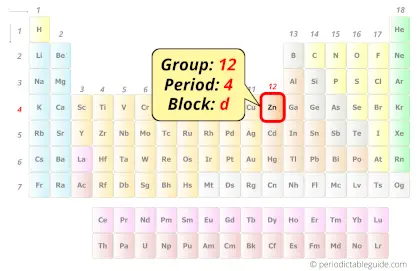 Group: 12, Period: 4, Block: d |
| Category | 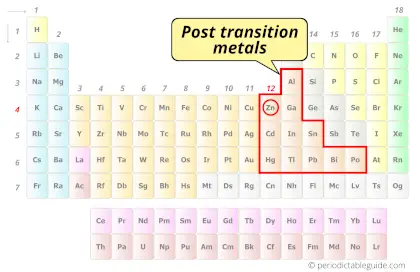 Post transition elements |
| Atomic number or Protons | 30 |
| Neutrons | 35 |
| Electrons | 30 |
| Symbol | Zn |
| Atomic mass |  65.38 u |
| Electrons arrangement or Bohr model | 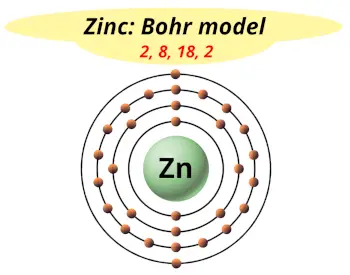 2, 8, 18, 2 |
| Electronic configuration | [Ar] 3d10 4s2 |
| Atomic radius | 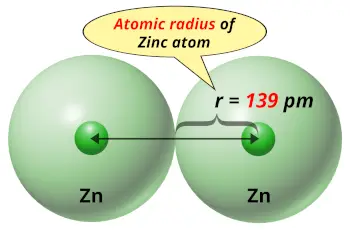 139 picometers (van der Waals radius) |
| 1st Ionization energy | 9.394 eV |
| Electronegativity | 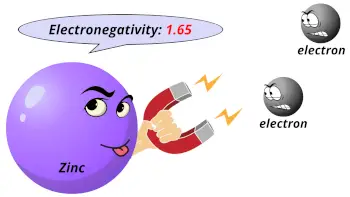 1.65 (Pauling scale) |
| Crystal structure | 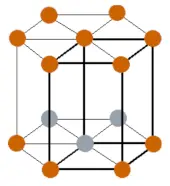 HCP (Hexagonal close packing) |
| Melting point | 692.6 K or 419.5 °C or 787.15 °F |
| Boiling point | 1180 K or 907 °C or 1665 °F |
| Density | 7.14 g/cm3 |
| Main isotope | 64Zn |
| CAS number | 7440-66-6 |
Zinc in Periodic table
Zinc element is in group 12 and period 4 of the Periodic table. Zinc is the d-block element and it belongs to Post transition elements group.
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba | La* | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| *Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| **Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
←Move to: Copper (Cu) element – Periodic Table
→Move to: Gallium (Ga) element – Periodic Table
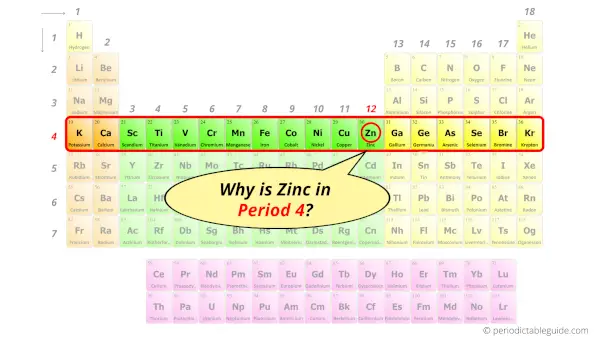
Why is Zinc in Period 4?
Let me ask you a question.
How many shells does zinc have?
It’s 4. Right?
You have already seen the bohr model of zinc atom in the above table.
From the Bohr model, it can be found that the number of orbits or shells in zinc is 4. Hence, as zinc has 4 orbits, it lies in period 4 of the Periodic table.
Why is Zinc in d-block?
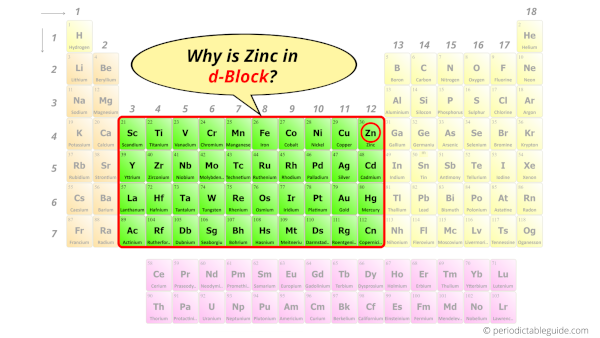
Before knowing this reason, first of all I want to ask you a simple question.
How can you determine the blocks-wise position of elements?
The simple answer: The elements will lie in the s, p, d or f block will completely depend upon the subshell in which the last electron will enter.
For example; the electron configuration of zinc is [Ar] 4s2 3d10.
So the last electron of zinc enters the d-subshell or d-orbital.
Hence, zinc is the d-block element.
Is Zinc a Transition Metal? Why?
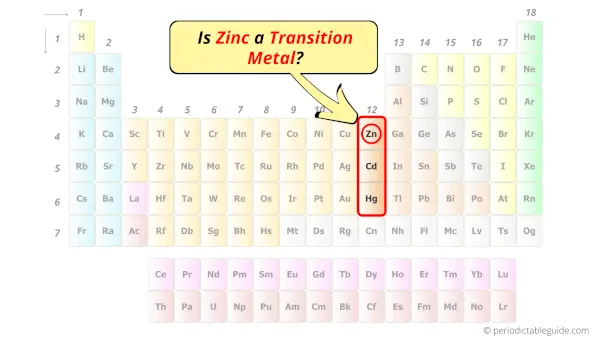
No, Zinc is not a transition metal because it has completely filled d-orbital in its ground state.
Let me explain the exact meaning of this.
According to the definition of transition metals;
The element should compulsorily have incomplete d-orbitals, either in their ground state (M) or most common oxidation states (M1+, M2+, etc) then only they are called transition metals.
Now, the ground state of Zinc means its normal state in which it has neither gained nor lost any electron/s.
So the ground state of Zinc is Zn.
And the ground state electronic configuration of Zinc is [Ar] 4s2 3d10.
In this state, if we see the electron configuration of Zinc, then it possesses 10 electrons in d-orbitals.
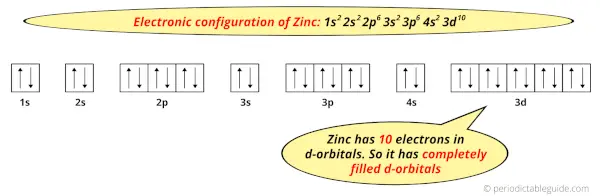
This indicates that the d-orbitals of zinc are completely filled.
And hence, as Zinc has completely filled d-orbitals, it is not considered as a transition metal.
9 Interesting facts about Zinc
Interesting facts about zinc element are mentioned below.
- The name zinc was derived from the German word “zinke” which means “pointed”.
- Zinc is the 24th most abundant element found from the earth’s crust.
- Zinc is the 2nd most abundant trace metal present in the human body after iron.
- Zinc is considered as the 4th common metal in the metallurgy industries after iron, aluminum and copper.
- According to Indian zinc associations, zinc is considered to be discovered by Indian metallurgists.
- Out of the total zinc produced nowadays, 50% of zinc is used in galvanisation.
- Around 17% of the total zinc production is used in the production of alloys like bronze and brass.
- Most of the zinc produced today comes from its ore zinc sulphide.
- Zinc is a recyclable metal and almost 30% of zinc that is produced today is recycled.
Properties of Zinc
The physical and chemical properties of zinc element are mentioned below.
Physical properties of Zinc
Physical properties of zinc are mentioned below.
- Zinc is a solid metal at STP and it is silvery grey in appearance.
- Atomic mass of zinc is 65.38 u and its density is 7.14 g/cm3.
- The melting point of zinc is 419.5 °C and its boiling point is 907 °C.
- Zinc has a less boiling point than iron or copper and hence it was not discovered earlier due to its boiling during the extraction of other metals.
- Zinc is a hard metal but it can be malleable above 100 °C.
- Zinc has many isotopes, but out of these isotopes, 64Zn is the most abundant (around 49%).
Chemical properties of Zinc
Chemical properties of zinc are mentioned below.
- Zinc is a less reactive metal and it is a strong reducing agent.
- The electron configuration of zinc shows completely filled d-orbitals, hence it is not considered as a transition metal. But it is classified as a post transition metal on the periodic table.
- The salts of zinc burn with bluish-green flame.
- Zinc reacts with air and forms a thin coating of zinc oxide which is dull-grey in color.
- Zinc is called an amphoteric metal as it reacts with both acid and alkalis.
Uses of Zinc
Uses of zinc are mentioned below.
- The main use of zinc is in galvanization. (Galvanization is the process of applying a thin layer of zinc on steel or iron to protect it from corrosion).
- Zinc is also used as an alloying element with other metals to get different properties.
- Zinc is also a necessary mineral element for the human body and its deficiency can hamper the immune system.
- Zinc is also present in products like sunscreens, paints, etc.
- Zinc is also present in batteries.
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
External resources:
- Zinc – Element information, properties and uses | Periodic Table. (n.d.). Zinc – Element Information, Properties and Uses | Periodic Table. https://www.rsc.org/periodic-table/element/30/zinc
- Zinc – Wikipedia. (2014, April 25). Zinc – Wikipedia. https://en.wikipedia.org/wiki/Zinc
- P. (n.d.). Zinc | Zn (Element) – PubChem. Zinc | Zn (Element) – PubChem. https://pubchem.ncbi.nlm.nih.gov/element/Zinc
- It’s Elemental – The Element Zinc. (n.d.). It’s Elemental – the Element Zinc. https://education.jlab.org/itselemental/ele030.html
