This is a SUPER easy guide on Bromine element.
In fact, the table mentioned below is the perfect information box (Which gives you every single detail about the Bromine element in Periodic table.)
So if you want to know anything about Bromine element, then this guide is for you.
Let’s dive right into it!
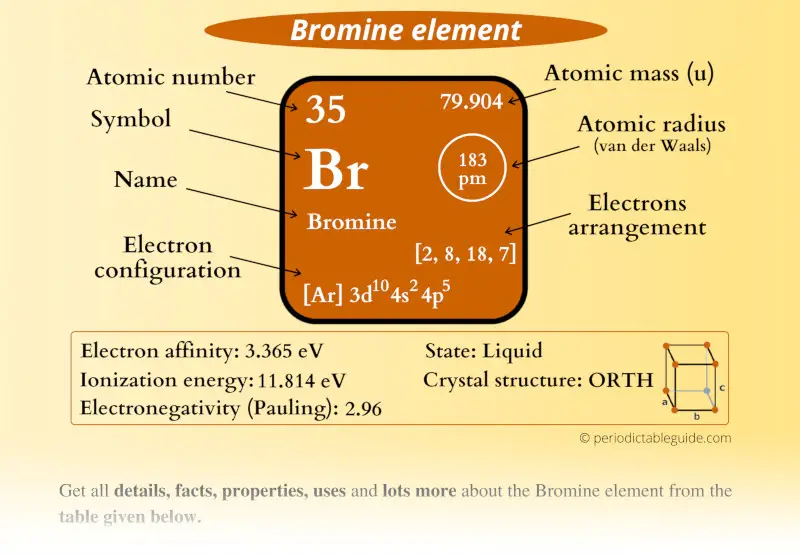
Bromine Element (Br) Information
| Appearance |  Reddish brown |
| State (at STP) | Liquid |
| Position in Periodic table | 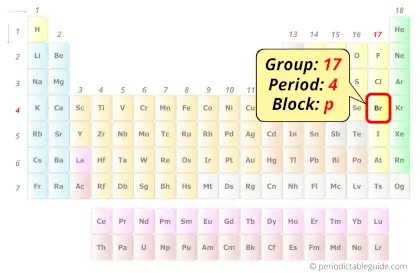 Group: 17, Period: 4, Block: p |
| Category | 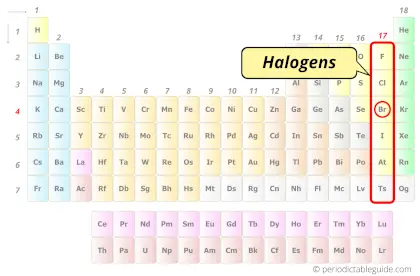 Halogens |
| Atomic number or Protons | 35 |
| Neutrons | 45 |
| Electrons | 35 |
| Symbol | Br |
| Atomic mass | 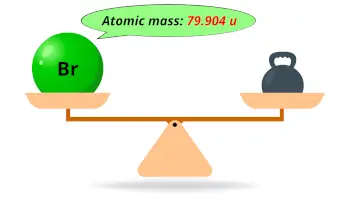 79.904 u |
| Electrons arrangement or Bohr model | 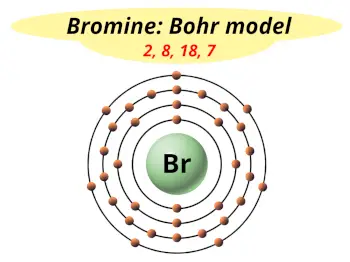 2, 8, 18, 7 |
| Electronic configuration | [Ar] 3d10 4s2 4p5 |
| Atomic radius | 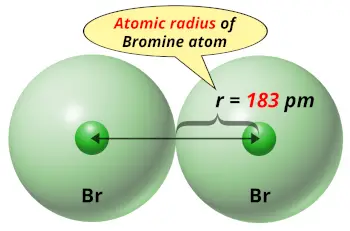 183 picometers (van der Waals radius) |
| Valence electrons | 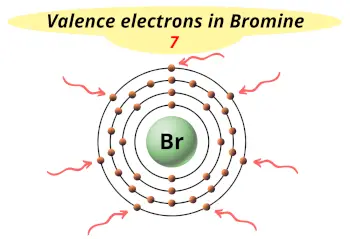 5 |
| 1st Ionization energy | 13.61 eV |
| Electronegativity |  2.96 (Pauling scale) |
| Crystal structure | 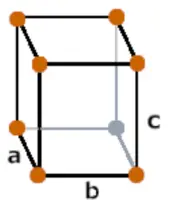 Orthorhombic |
| Melting point | 265.8 K or -7.2 °C or 19 °F |
| Boiling point | 332 K or 58.8 °C or 137.8 °F |
| Density | 3.12 g/cm3 |
| Main isotope | 79Br (51%) and 81Br (49%) |
| Who discovered Bromine and when? |  Antoine Jerome Balard and Carl Jacob Lowig (in 1825) |
| CAS number | 7726-95-6 |
Bromine in Periodic table
Bromine element is in group 17 and period 4 of the Periodic table. Bromine is the p-block element and it belongs to halogens group.
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba | La* | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| *Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| **Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
←Move to: Selenium (Se) element – Periodic Table
→Move to: Krypton (Kr) element – Periodic Table
Why is Bromine in Group 17?
Do you know, how many electrons can be accommodated in the first shell, second shell, third shell, fourth shell, etc…?
Here is the table showing the capacity of orbits to hold electrons.
Number of electrons in shells.
| Orbit / Shell (n) | Maximum no. of electrons this orbit can hold |
| K shell, n = 1 | 2 × 1² = 2 |
| L shell, n = 2 | 2 × 2² = 8 |
| M shell, n = 3 | 2 × 3² = 18 |
| N shell, n = 4 | 2 × 4² = 32 |
Thus,
- 1st shell can hold 2 electrons.
- 2nd shell can hold 8 electrons.
- 3rd shell can hold 18 electrons.
- 4th shell can hold 32 electrons.
Now the atomic number of bromine (Br) is 35.
Hence the bromine element has electrons arrangement 2, 8, 18, 7.
This electron arrangement indicates that the outermost orbit of Bromine element (Br) has 7 electrons.
Hence, it lies in group 17.
Why is Bromine in Period 4?
Let me ask you a question.
How many shells does bromine have?
It’s 4. Right?
You have already seen the bohr model of bromine atom in the above table.
From the Bohr model, it can be found that the number of orbits or shells in bromine is 4. Hence, as bromine has 4 orbits, it lies in period 4 of the Periodic table.
Why is Bromine in p-block?
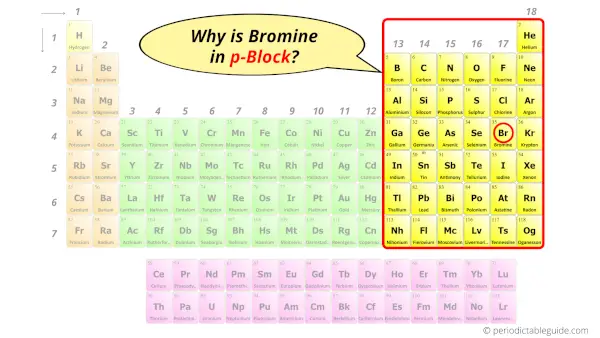
Before knowing this reason, first of all I want to ask you a simple question.
How can you determine the blocks-wise position of elements?
The simple answer: The elements will lie in the s, p, d or f block will completely depend upon the subshell in which the last electron will enter.
For example; the electron configuration of bromine is [Ar] 3d10 4s2 4p5.
So the last electron of bromine enters the p-subshell or p-orbital.
Hence, bromine is the p-block element.
9 Interesting facts about Bromine
Interesting facts about bromine element are mentioned below.
- Bromine is the only nonmetal that exists in a liquid form at room temperature.
- Bromine and mercury are the only elements that are in liquid state at standard temperature and pressure.
- The name “bromine” was derived from the Greek word “bromos”.
- Bromine is the 44th most abundant element found from the earth’s crust.
- The abundance of bromine in the earth’s crust is around 2.4 ppm by weight.
- Out of all the elements of halogen group, bromine is the 3rd lightest element.
- Bromine is approximately 3 times denser than water.
- Bromine atoms are more destructive to the ozone layer as compared to that of chlorine atoms.
- Pure bromine is a toxic element and it can also cause burns on skin.
Properties of Bromine
The physical and chemical properties of bromine element are mentioned below.
Physical properties of Bromine
Physical properties of bromine are mentioned below.
- Bromine is a nonmetal in liquid state having a reddish brown color.
- The bromine vapors have a very pungent odour and it can also irritate our eyes.
- The melting point of bromine is -7.2 °C and its boiling point is 58.8 °C.
- The atomic mass of bromine is 79.904 u and its density is 3.12 g/cm3.
- Bromine has a very less number of isotopes. And out of them, only 2 isotopes are stable (79Br and 81Br). 79Br has an abundance of 51% and 81Br has an abundance of 49%.
Chemical properties of Bromine
Chemical properties of bromine are mentioned below.
- Bromine is a reactive nonmetal and hence it is not found in a free state in nature.
- The electron arrangement in bromine atom is 2, 8, 18, 7. Hence, most commonly the bromine atom loses one electron during a chemical reaction.
- The most common oxidation state of bromine is -1.
- Bromine reacts with metals and produces salts.
- Pure bromine exists as a diatomic molecule (i.e Br2).
Uses of Bromine
Uses of bromine are mentioned below.
- Bromine is used in manufacturing insecticides as well as many other chemicals used in agriculture.
- Bromine is also used for water purification as well as in manufacturing sanitizers.
- Bromine is also used in swimming pools as an alternative to chlorine.
- Bromine is widely used in making of flame retardant material.
- Silver bromide is a compound containing bromine element that is used in photography.
- Bromine was also used as a dye material in ancient times.
Free Gift for you: Interactive Periodic Table
Let me tell you how this Interactive Periodic Table will help you in your studies.
1). You can effortlessly find every single detail about the elements from this single Interactive Periodic table.
2). You will get the detailed information about the periodic table which will convert a newbie into pro.
3). You will also get the HD images of the Periodic table (for FREE).
Checkout Interactive Periodic table and download it’s high resolution image now (It’s FREE)
External resources:
- Bromine – Wikipedia. (2016, January 5). Bromine – Wikipedia. https://en.wikipedia.org/wiki/Bromine
- It’s Elemental – The Element Bromine. (n.d.). It’s Elemental – the Element Bromine. https://education.jlab.org/itselemental/ele035.html
- P. (n.d.). Bromine. Bromine | Br2 – PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/24408
- CDC | Facts About Bromine. (2018, April 4). CDC | Facts About Bromine. https://emergency.cdc.gov/agent/bromine/basics/facts.asp
- Bromine – Element information, properties and uses | Periodic Table. (n.d.). Bromine – Element Information, Properties and Uses | Periodic Table. https://www.rsc.org/periodic-table/element/35/bromine