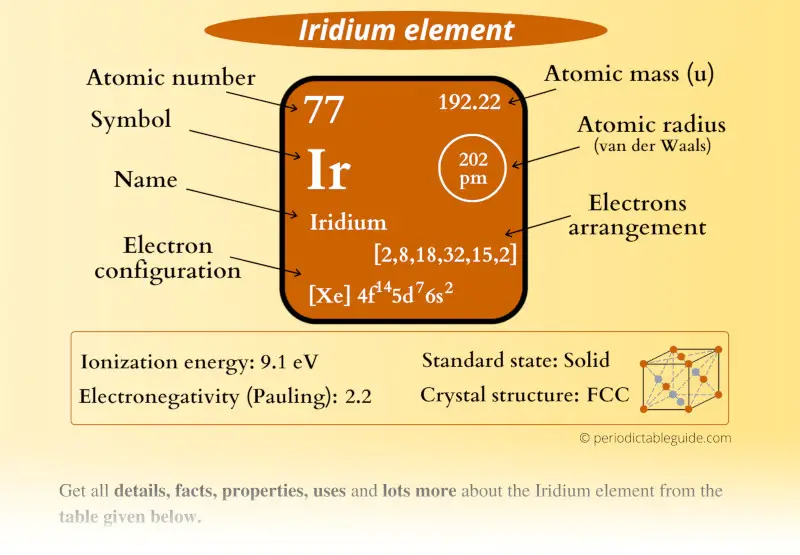
This is a SUPER easy guide on Iridium element.
In fact, the table mentioned below is the perfect information box (Which gives you every single detail about the Iridium element in Periodic table.)
So if you want to know anything about Iridium element, then this guide is for you.
Let’s finish this very quickly.
Iridium Element (Ir) Information
| Appearance |  Silvery white metallic appearance |
| State (at STP) | Solid |
| Position in Periodic table | 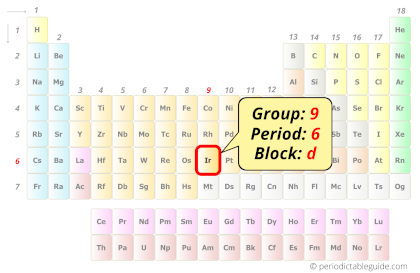 Group: 9, Period: 6, Block: d |
| Category | 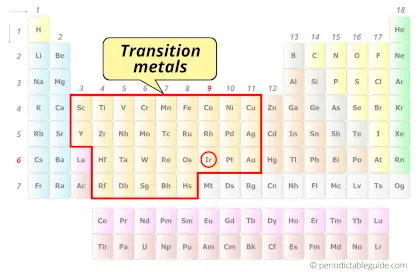 Transition metals |
| Atomic number or Protons | 77 |
| Neutrons | 115 |
| Electrons | 77 |
| Symbol | Ir |
| Atomic mass | 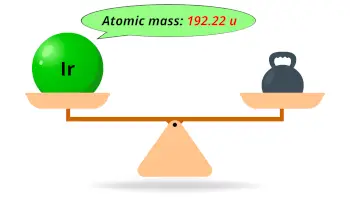 192.22 u |
| Electrons arrangement or Bohr model | 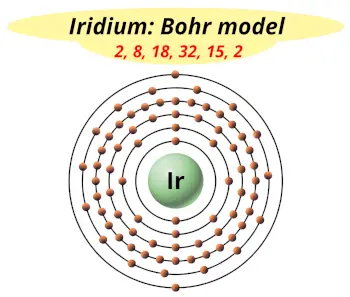 2, 8, 18, 32, 15, 2 |
| Electronic configuration | [Xe] 4f14 5d7 6s2 |
| Atomic radius | 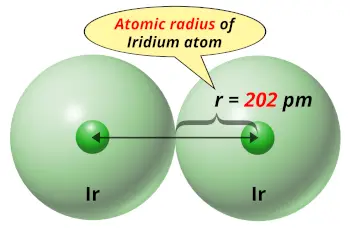 202 picometers (van der Waals radius) |
| 1st Ionization energy | 9.1 eV |
| Electronegativity | 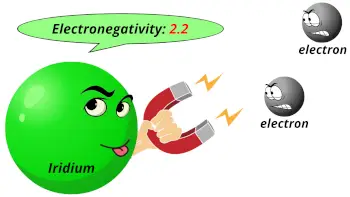 2.2 (Pauling scale) |
| Crystal structure | 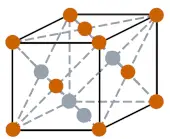 FCC (Face centered cubic) |
| Melting point | 2719 K or 2446 °C or 4435 °F |
| Boiling point | 4403 K or 4130 °C or 7466 °F |
| Density | 22.56 g/cm3 |
| Main isotope | 193Ir |
| Who discovered Iridium and when? | Smithson Tennant in 1803 |
| CAS number | 7439-88-5 |
Iridium in Periodic table
Iridium element is in group 9 and period 6 of the Periodic table. Iridium is the d-block element and it belongs to transition metals group.
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba | La* | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| *Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| **Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
←Move to: Osmium (Os) element – Periodic Table
→Move to: Platinum (Pt) element – Periodic Table
Why is Iridium in Period 6?
Let me ask you a question.
How many shells does an iridium atom have?
It’s 6. Right?
You have already seen the bohr model of iridium atom in the above table.
From the Bohr model, it can be found that the number of orbits or shells in iridium is 6. Hence, as iridium has 6 orbits, it lies in period 6 of the Periodic table.
Why is Iridium in d-block?
Before knowing this reason, first of all I want to ask you a simple question.
How can you determine the blocks-wise position of elements?
The simple answer: The elements will lie in the s, p, d or f block will completely depend upon the subshell in which the last electron will enter.
For example; the electron configuration of iridium is [Xe] 4f14 5d7 6s2.
So the last electron of iridium enters the d-subshell or d-orbital.
Hence, iridium is the d-block element.
Is Iridium a Transition Metal? Why?
Yes, Iridium is a transition metal because it has incompletely filled d-orbital in its ground state.
Let me explain the exact meaning of this.
According to the definition of transition metals;
The element should compulsorily have incomplete d-orbitals, either in their ground state (M) or most common oxidation states (M1+, M2+, etc) then only they are called transition metals.
Now, the ground state of Iridium means its normal state in which it has neither gained nor lost any electron/s.
So the ground state of Iridium is Ir.
And the ground state electronic configuration of Iridium is [Xe] 4f14 5d7 6s2.
In this state, if we see the electron configuration of Iridium, then it possesses incomplete d-orbitals.
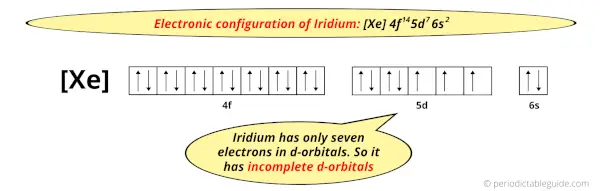
Because, there are only seven electrons in the d-orbitals (here 5d orbitals).
In order to have the complete d-orbitals, there must be 10 electrons in it.
But in the ground state electronic configuration of iridium, you can see that it has only 7 electrons in d-orbitals.
Thus, Iridium has incomplete d-orbitals.
And hence, as Iridium has incomplete d-orbitals, it is considered as a transition metal.
5 Interesting facts about Iridium
Interesting facts about iridium element are mentioned below.
- The name “Iridium” was derived from the Latin word “Iris”, which means rainbow.
- Iridium was discovered by Chemist Smithson Tennant in 1803.
- Iridium is available in very rare quantities in the earth’s crust. Its concentration in earth’s crust is approximately 0.4 parts per billion.
- Iridium is the 2nd most dense metal on the periodic table after osmium.
- It is very difficult to machine iridium metal as it is very hard.
Properties of Iridium
The physical and chemical properties of iridium element are mentioned below.
Physical properties of Iridium
Physical properties of iridium are mentioned below.
- Iridium is a very dense metal having a silvery white metallic appearance.
- The melting point of iridium is 2446 °C and its boiling point is 4130 °C.
- The crystal structure of iridium is FCC (Face centered cubic).
- The atomic mass of iridium is 192.22 u and its density is 22.56 g/cm3.
- There are many isotopes of iridium, but out of those isotopes, the most abundant isotope is 193Ir (having an abundance of approximately 62.7%).
Chemical properties of Iridium
Chemical properties of iridium are mentioned below.
- Iridium is chemically unreactive metal and it is resistive to almost all the acids.
- Iridium is reactive to salts like NaCl (sodium chloride) and NaCN (sodium cyanide).
- At higher temperatures, iridium may be attacked by oxygen, but not at room temperature.
- Iridium when reacts with sulfur, it gives iridium disulfide.
- The electron configuration of iridium is [Xe] 4f14 5d7 6s2, in which the last electron enters the d-orbital. Thus iridium is a transition metal.
Uses of Iridium
Uses of iridium are mentioned below.
- As Iridium is a dense metal, it is used as an alloying element to make the alloys hard.
- Iridium can resist higher temperatures and hence it is also used in crucibles.
- The international standard kilogram weight was made of platinum iridium alloy. It contains 90% platinum and 10% iridium.
- Iridium is also used in making fountain pen tips as well as it is also used in making electrical contacts.
Free Gift for you: Interactive Periodic Table
Let me tell you how this Interactive Periodic Table will help you in your studies.
1). You can effortlessly find every single detail about the elements from this single Interactive Periodic table.
2). You will get the detailed information about the periodic table which will convert a newbie into pro.
3). You will also get the HD images of the Periodic table (for FREE).
Checkout Interactive Periodic table and download it’s high resolution image now (It’s FREE)
External resources:
- Iridium – Wikipedia. (2023, January 1). Iridium – Wikipedia. https://en.wikipedia.org/wiki/Iridium
- Iridium – Element information, properties and uses | Periodic Table. (n.d.). Iridium – Element Information, Properties and Uses | Periodic Table. https://www.rsc.org/periodic-table/element/77/iridium
- P. (n.d.). Iridium | Ir (Element) – PubChem. Iridium | Ir (Element) – PubChem. https://pubchem.ncbi.nlm.nih.gov/element/Iridium
- It’s Elemental – The Element Iridium. (n.d.). It’s Elemental – the Element Iridium. https://education.jlab.org/itselemental/ele077.html