This is a SUPER easy guide on Bohrium element.
In fact, the table mentioned below is the perfect information box (Which gives you every single detail about the Bohrium element in Periodic table.)
So if you want to know anything about Bohrium element, then this guide is for you.
Let’s dive right into it!
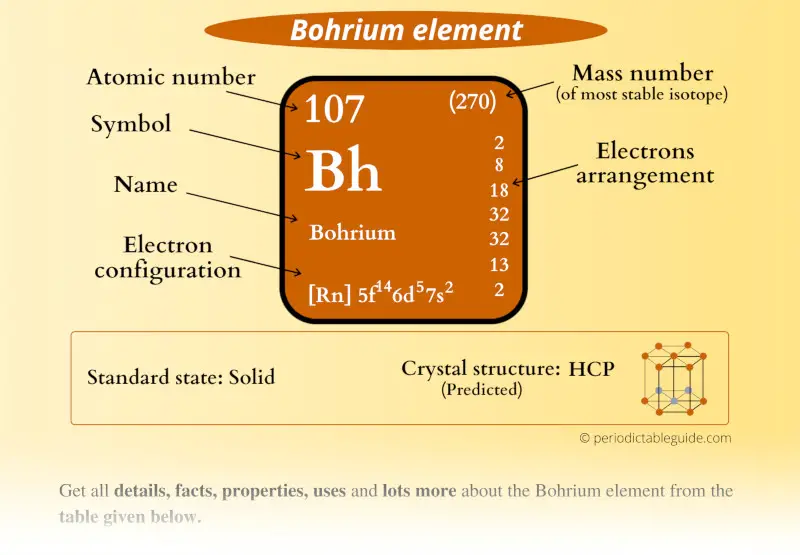
Bohrium Element (Bh) Information
| Position in Periodic table | 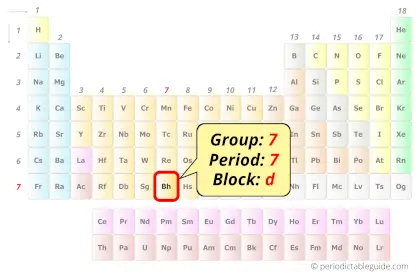 Group: 7, Period: 7, Block: d |
| Category | 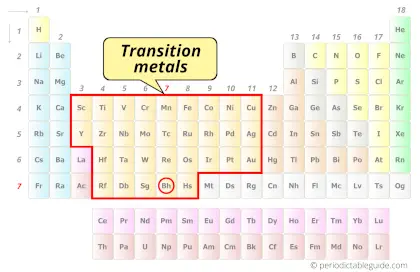 Transition metals |
| Atomic number or Protons | 107 |
| Neutrons | 155 |
| Electrons | 107 |
| Symbol | Bh |
| Atomic mass of Bohrium (most stable isotope) | 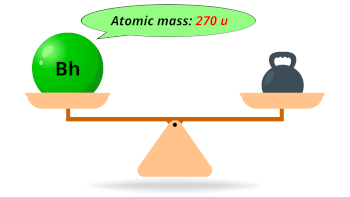 270 u |
| Electrons arrangement or Bohr model | 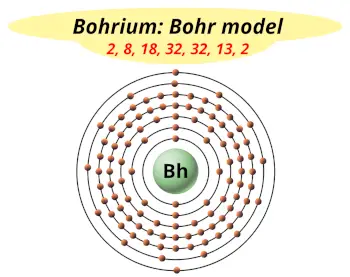 2, 8, 18, 32, 32, 13, 2 |
| Electronic configuration | [Rn] 5f14 6d5 7s2 |
| Crystal structure (predicted) | 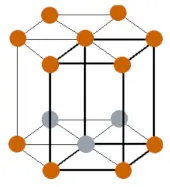 HCP (hexagonal close packed) |
| Density (predicted) | 26-27 g/cm3 |
| CAS number | 54037-14-8 |
Bohrium in Periodic table
Bohrium element is in group 7 and in period 7 of the Periodic table. Bohrium is the d-block element and it belongs to transition metals group.
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba | La* | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| *Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| **Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
←Move to: Seaborgium (Sg) element – Periodic Table
→Move to: Hassium (Hs) element – Periodic Table
Why is Bohrium in Period 7?
Let me ask you a question.
How many shells does a bohrium atom have?
It’s 7. Right?
You have already seen the bohr model of bohrium atom in the above table.
From the Bohr model, it can be found that the number of orbits or shells in bohrium is 7. Hence, as bohrium has 7 orbits, it lies in period 7 of the Periodic table.
Why is Bohrium in d-block?
Before knowing this reason, first of all I want to ask you a simple question.
How can you determine the blocks-wise position of elements?
The simple answer: The elements will lie in the s, p, d or f block will completely depend upon the subshell in which the last electron will enter.
For example; the electron configuration of bohrium is [Rn] 5f14 6d5 7s2.
So the last electron of bohrium enters the d-subshell or d-orbital.
Hence, bohrium is the d-block element.
5 facts about Bohrium
Interesting facts about bohrium element are mentioned below.
- The element was given the name “Bohrium” to honor the physicist Neils Bohr.
- Bohrium element was discovered by Peter Armbruster and his team in 1981, at Darmstadt, Germany.
- Bohrium is not found naturally, but it is artificially prepared in the laboratory.
- There are around 12 isotopes of bohrium and all are radioactive in nature.
- The most stable isotope of bohrium is 270Bh, which has a half life of only 61 seconds.
Properties of Bohrium
The physical and chemical properties of bohrium element are mentioned below.
- Bohrium is a radioactive element which has a very short half life (few seconds).
- Bohrium is expected to have a solid phase at STP.
- The calculated atomic mass of the most stable isotope of bohrium is 270 u and its density is predicted to be 26-27 g/cm3.
- The predicted crystal structure of bohrium is HCP (i.e hexagonal close packed).
- The common oxidation state of bohrium is expected to be +7, but it may show other oxidation states like +3, +4 and +5.
Uses of Bohrium
Bohrium is generally used for research work in chemistry. There is no commercial use of bohrium due to its radioactive nature and very little half-life.
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
External resources:
- Bohrium – Element information, properties and uses | Periodic Table. (n.d.). Bohrium – Element Information, Properties and Uses | Periodic Table. https://www.rsc.org/periodic-table/element/107/bohrium
- Bohrium – Wikipedia. (2013, October 4). Bohrium – Wikipedia. https://en.wikipedia.org/wiki/Bohrium
- P. (n.d.). Bohrium | Bh (Element) – PubChem. Bohrium | Bh (Element) – PubChem. https://pubchem.ncbi.nlm.nih.gov/element/Bohrium
- It’s Elemental – The Element Bohrium. (n.d.). It’s Elemental – the Element Bohrium. https://education.jlab.org/itselemental/ele107.html
- Eichler, R., Brüchle, W., Dressler, R., Düllmann, C., Eichler, B., Gäggeler, H. W., Gregorich, K. E., Hoffman, D. C., Hübener, S., Jost, D. T., Kirbach, U. W., Laue, C. A., Lavanchy, V. M., Nitsche, H., Patin, J. B., Piguet, D., Schädel, M., Shaughnessy, D. A., Strellis, D. A., . . . Yakushev, A. B. (2000, September). Chemical characterization of bohrium (element 107). Nature, 407(6800), 63–65. https://doi.org/10.1038/35024044
