This is a SUPER easy guide on Tin element.
In fact, the table mentioned below is the perfect information box (Which gives you every single detail about the Tin element in Periodic table.)
So if you want to know anything about Tin element, then this guide is for you.
Let’s dive right into it!
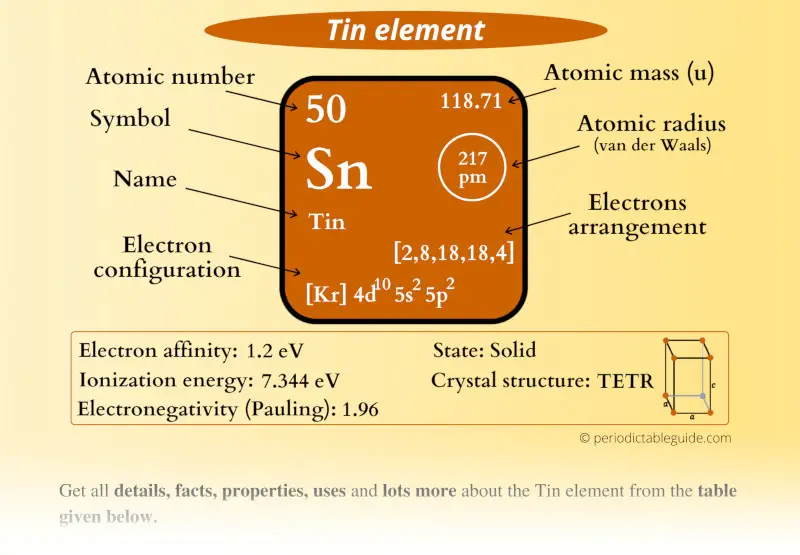
Tin Element (Sn) Information
| Appearance |  Silvery white or gray in color |
| State (at STP) | Solid |
| Position in Periodic table | 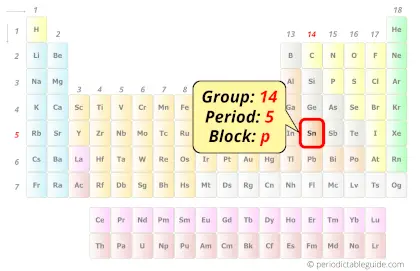 Group: 14, Period: 5, Block: p |
| Category | 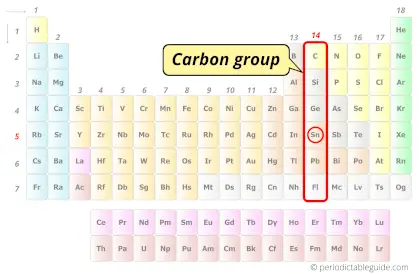 Carbon group |
| Atomic number or Protons | 50 |
| Neutrons | 69 |
| Electrons | 50 |
| Symbol | Sn |
| Atomic mass | 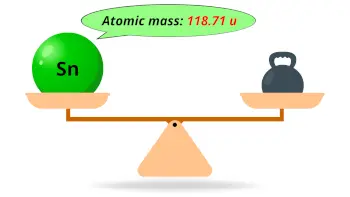 118.71 u |
| Electrons arrangement or Bohr model | 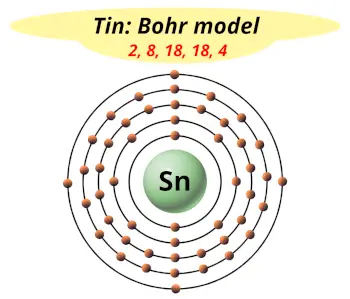 2, 8, 18, 18, 4 |
| Electronic configuration | [Kr] 4d10 5s2 5p2 |
| Atomic radius | 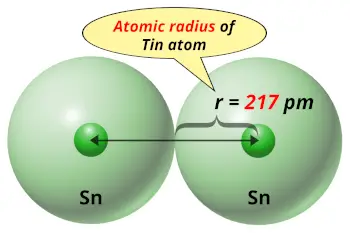 217 picometers (van der Waals radius) |
| Valence electrons | 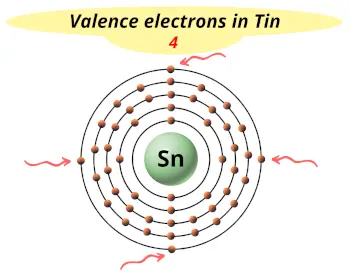 4 |
| 1st Ionization energy | 7.344 eV |
| Electronegativity | 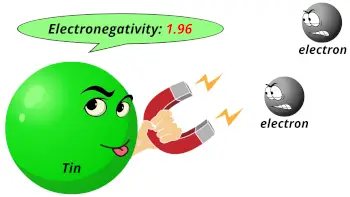 1.96 (Pauling scale) |
| Crystal structure | 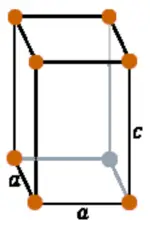 Tetragonal |
| Melting point | 505.1 K or 231.9 °C or 449.4 °F |
| Boiling point | 2875 K or 2602 °C or 4716 °F |
| Density | 7.31 g/cm3 |
| Main isotope | 120Sn |
| CAS number | 7440-31-5 |
Tin in Periodic table
Tin element is in group 14 and period 5 of the Periodic table. Tin is the p-block element and it belongs to carbon group.
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba | La* | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| *Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| **Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
←Move to: Indium (In) element – Periodic Table
→Move to: Antimony (Sb) element – Periodic Table
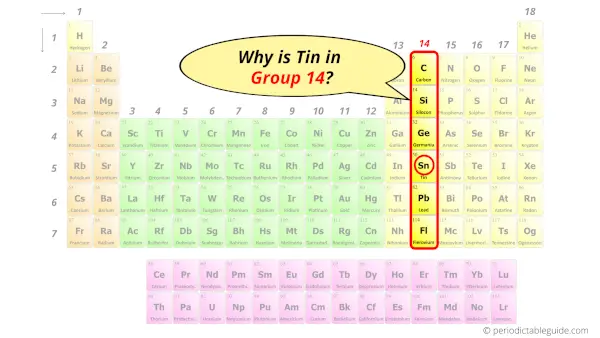
Why is Tin in Group 14?
Do you know, how many electrons can be accommodated in the first shell, second shell, third shell, fourth shell, etc…?
Here is the table showing the capacity of orbits to hold electrons.
Number of electrons in shells.
| Orbit / Shell (n) | Maximum no. of electrons this orbit can hold (2 × n2) |
| K shell, n = 1 | 2 × 1² = 2 |
| L shell, n = 2 | 2 × 2² = 8 |
| M shell, n = 3 | 2 × 3² = 18 |
| N shell, n = 4 | 2 × 4² = 32 |
| . . . | . . . |
Thus,
- 1st shell can hold 2 electrons.
- 2nd shell can hold 8 electrons.
- 3rd shell can hold 18 electrons.
- 4th shell can hold 32 electrons, and so on.
Now the atomic number of Tin (Sn) is 50.
Hence the electron arrangement in Tin is 2, 8, 18, 18, 4. And the electron configuration of Tin is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2.
This electron arrangement and electron configuration indicates that the outermost orbit (i.e orbit number 5) of Tin atom has 4 electrons.
Hence, it lies in group 14.
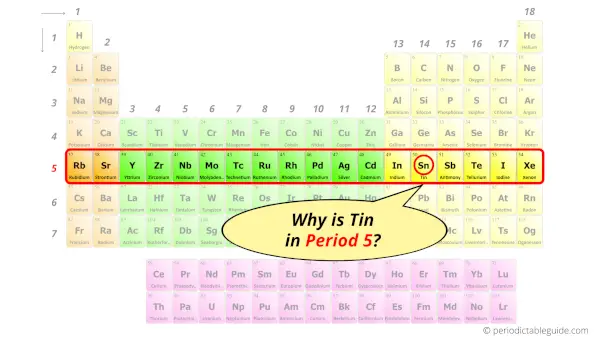
Why is Tin in Period 5?
Let me ask you a question.
How many shells does tin have?
It’s 5. Right?
You have already seen the bohr model of tin atom in the above table.
From the Bohr model, it can be found that the number of orbits or shells in a tin atom is 5. Hence, as the tin atom has 5 orbits, it lies in period 5 of the Periodic table.
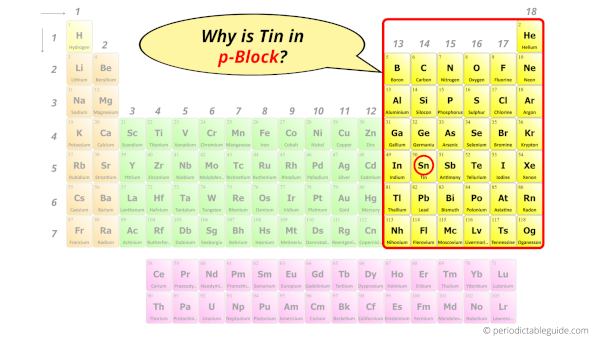
Why is Tin in p-block?
Before knowing this reason, first of all I want to ask you a simple question.
How can you determine the blocks-wise position of elements?
The simple answer: The elements will lie in the s, p, d or f block will completely depend upon the subshell in which the last electron will enter.
For example; the electron configuration of tin is [Kr] 4d10 5s2 5p2.
So the last electron of tin enters the p-subshell or p-orbital.
Hence, tin is the p-block element.
5 Interesting facts about Tin
Interesting facts about tin element are mentioned below.
- Tin makes up around 2 ppm (parts per million) of the earth’s crust.
- Tin is the 50th most abundant element found from the earth’s crust.
- Tin (Sn) is obtained from its various ores, but its main ore is SnO2.
- When the tin is bent, it makes a screaming sound. This creaking sound occurs due to deformation of tin crystals. And this sound is known as “Tin cry”.
- The isotopes of tin are more stable than the isotopes of any other elements.
Properties of Tin
The physical and chemical properties of tin element are mentioned below.
Physical properties of Tin
Physical properties of tin are mentioned below.
- At room temperature, tin is a soft metal having silvery white or grey color.
- Tin is a malleable metal and hence it can be drawn into thin sheets.
- When tin is polished, it produces a shiny surface.
- The atomic mass of tin is 118.71 u and its density is 7.31 g/cm3.
- The melting point of tin is 231.9 °C and its boiling point is 2602 °C.
- The crystal structure of tin is tetragonal.
- Tin has many stable isotopes, but out of them 120Sn is the most abundant (around 32.5%).
Chemical properties of Tin
Chemical properties of tin are mentioned below.
- Below 13 °C, tin turns into its allotrope called “alpha tin”, and it behaves as a semiconductor.
- Tin is a corrosion resistance metal. It does not even react with water.
- Tin is alloyed with copper to produce a new alloy called bronze. In this alloy the proportion of tin is 12% and that of copper is 88%.
- The first ionization energy of tin is 7.344 eV.
- The electronegativity of tin is 1.96 on the Pauling scale.
Uses of Tin
Uses of tin are mentioned below.
- The main use of tin is in the manufacturing of bronze (which is an alloy of tin and copper).
- The solder which is used in the soldering process is made up of tin and lead alloy.
- As tin is a corrosion resistance metal, it is used for plating other metals.
- As tin has a lower melting point, it is also used in manufacturing fusible plugs.
- The crystalline tin-niobium alloys behave as a superconductor at very low temperatures.
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
External resources:
- Periodic Table of Elements: Los Alamos National Laboratory. (n.d.). Periodic Table of Elements: Los Alamos National Laboratory. https://periodic.lanl.gov/50.shtml
- Tin – Element information, properties and uses | Periodic Table. (n.d.). Tin – Element Information, Properties and Uses | Periodic Table. https://www.rsc.org/periodic-table/element/50/tin
- Tin – Wikipedia. (2013, January 23). Tin – Wikipedia. https://en.wikipedia.org/wiki/Tin
- P. (n.d.). Tin | Sn (Element) – PubChem. Tin | Sn (Element) – PubChem. https://pubchem.ncbi.nlm.nih.gov/element/Tin
- It’s Elemental – The Element Tin. (n.d.). It’s Elemental – the Element Tin. https://education.jlab.org/itselemental/ele050.html
