This is a SUPER easy guide on Thorium element.
In fact, the table mentioned below is the perfect information box (Which gives you every single detail about the Thorium element in Periodic table.)
So if you want to know anything about Thorium element, then this guide is for you.
Let’s dive right into it!
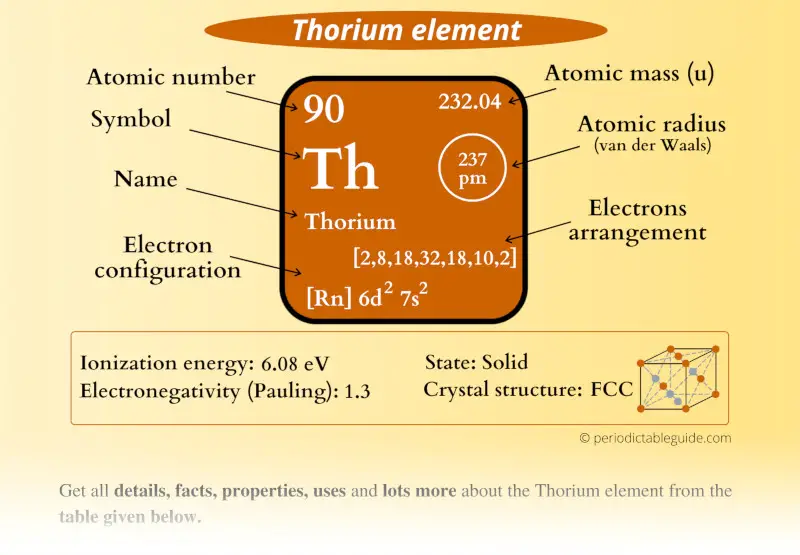
Thorium Element (Th) Information
| Appearance | Silvery-white metallic appearance |
| State (at STP) | Solid |
| Position in Periodic table | 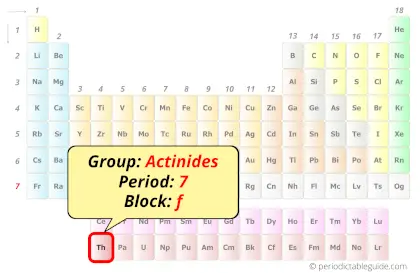 Group: actinides, Period: 7, Block: f |
| Category | 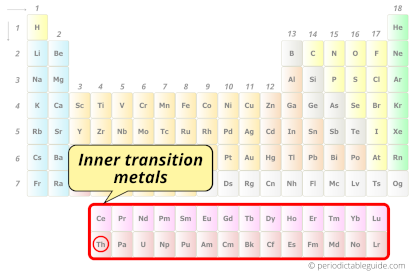 Inner transition metals |
| Atomic number or Protons | 90 |
| Neutrons | 142 |
| Electrons | 90 |
| Symbol | Th |
| Atomic mass | 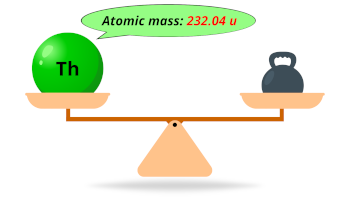 232.04 u |
| Electrons arrangement or Bohr model | 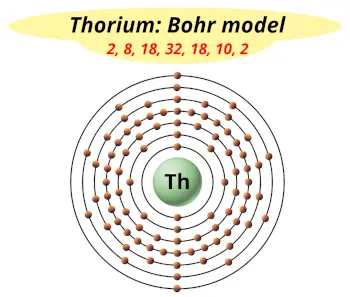 2, 8, 18, 32, 18, 10, 2 |
| Electronic configuration | [Rn] 6d2 7s2 |
| Atomic radius | 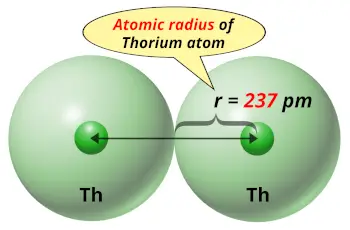 237 picometers (van der Waals radius) |
| 1st Ionization energy | 6.08 eV |
| Electronegativity | 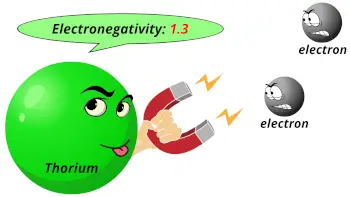 1.3 (Pauling scale) |
| Crystal structure | 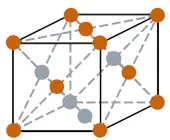 FCC (face centered cubic) |
| Melting point | 2023 K or 1750 °C or 3182 °F |
| Boiling point | 5061 K or 4788 °C or 8650 °F |
| Density | 11.725 g/cm3 |
| Main isotope | 232Th |
| Who discovered Thorium and when? |  Jons Jakob Berzelius (in 1829) |
| CAS number | 7440-29-1 |
Thorium in Periodic table
Thorium element is in period 7 and in actinides group of the Periodic table. Thorium is the f-block element and it belongs to inner transition metals group.
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba | La* | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| *Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| **Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
←Move to: Actinium (Ac) element – Periodic Table
→Move to: Protactinium (Pa) element – Periodic Table
Why is Thorium in Period 7?
Let me ask you a question.
How many shells does thorium have?
It’s 7. Right?
You have already seen the bohr model of thorium atom in the above table.
From the Bohr model, it can be found that the number of orbits or shells in thorium is 7. Hence, as thorium has 7 orbits, it lies in period 7 of the Periodic table.
5 Interesting facts about Thorium
Interesting facts about thorium element are mentioned below.
- The name “Thorium” was derived from “Thor” which is the name of the Norse god of thunder.
- The concentration of thorium in the earth’s crust is approximately 6 ppm by weight.
- The Thorium element was discovered by chemist Jons Jakob Berzelius in 1829.
- Thorium is mostly mined from regions of Australia, Canada, United states of America, Russia and India.
- Thorite, thorianite and monazite are the major minerals which contain thorium in higher concentrations.
Properties of Thorium
The physical and chemical properties of thorium element are mentioned below.
Physical properties of Thorium
Physical properties of thorium are mentioned below.
- Thorium is a solid metal having a silvery-white metallic appearance.
- The melting point of thorium is 1750 °C and its boiling point is 4788 °C.
- The crystal structure of thorium is FCC (face centered cubic).
- Thorium has many isotopes, but out of those isotopes, the most stable isotope is 232Th, which has a half-life of 14 billion years.
- The atomic mass of thorium is 232.04 u and its density is 11.725 g/cm3.
Chemical properties of Thorium
Chemical properties of thorium are mentioned below.
- Thorium reacts with atmospheric oxygen and it tarnishes slowly, which results in a black oxide layer on it.
- Thorium also reacts with hydrogen, sulfur and halogens like fluorine, chlorine, bromine, etc.
- The most common oxidation state of thorium is +4. But apart from this, it also exists in other oxidation states such as +1, +2 and +3.
- The powdered thorium metal is highly reactive and it can even ignite in air.
- The thorium metal shows a slow reaction with water.
- Thorium dissolves in hydrochloric acid.
Uses of Thorium
Uses of thorium are mentioned below.
- Thorium is used as an alloying element with magnesium to increase the strength of magnesium.
- Thorium dioxide (which is a compound of thorium) is added to glass to increase the refractive index of glass.
- Thorium dioxide is also used in manufacturing of ceramics that are resistant to higher temperatures.
- Nowadays, the commercial use of thorium is minimized due to its radioactive nature.
- The heat present inside the earth’s crust is because of thorium as well as uranium.
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
External resources:
- Thorium – Element information, properties and uses | Periodic Table. (n.d.). Thorium – Element Information, Properties and Uses | Periodic Table. https://www.rsc.org/periodic-table/element/90/thorium
- Thorium – Wikipedia. (2013, October 4). Thorium – Wikipedia. https://en.wikipedia.org/wiki/Thorium
- C&EN: IT’S ELEMENTAL: THE PERIODIC TABLE – THORIUM. (n.d.). C&EN: IT’S ELEMENTAL: THE PERIODIC TABLE – THORIUM. https://pubsapp.acs.org/cen/80th/thorium.html?
- It’s Elemental – The Element Thorium. (n.d.). It’s Elemental – the Element Thorium. https://education.jlab.org/itselemental/ele090.html
- Thorium – World Nuclear Association. (n.d.). Thorium – World Nuclear Association. https://world-nuclear.org/information-library/current-and-future-generation/thorium.aspx
- P. (n.d.). Thorium | Th (Element) – PubChem. Thorium | Th (Element) – PubChem. https://pubchem.ncbi.nlm.nih.gov/element/Thorium
- Thorium. (n.d.). Thorium. https://pubs.usgs.gov/of/2004/1050/thorium.htm
- Thorium | CCDC. (n.d.). Thorium | CCDC. https://www.ccdc.cam.ac.uk/elements/thorium/
