This is a SUPER easy guide on Terbium element.
In fact, the table mentioned below is the perfect information box (Which gives you every single detail about the Terbium element in Periodic table.)
So if you want to know anything about Terbium element, then this guide is for you.
Let’s finish this very quickly.
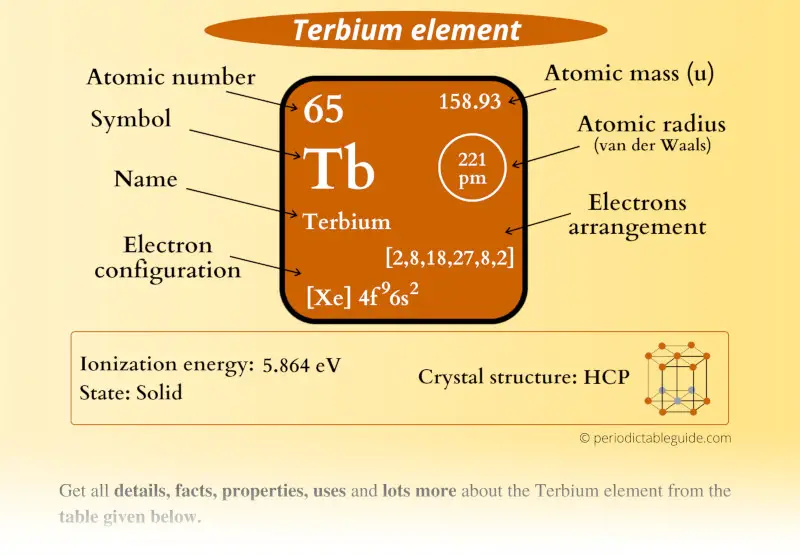
Terbium Element (Tb) Information
| Appearance |  Silvery white metallic surface |
| State (at STP) | Solid |
| Position in Periodic table | 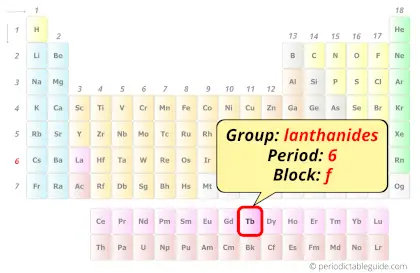 Group: lanthanides, Period: 6, Block: f |
| Category | 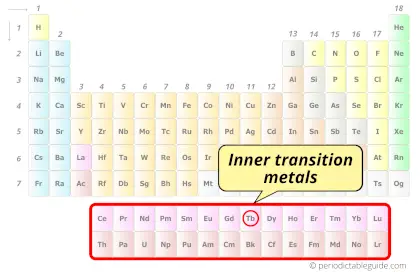 Inner transition metals |
| Atomic number or Protons | 65 |
| Neutrons | 94 |
| Electrons | 65 |
| Symbol | Tb |
| Atomic mass | 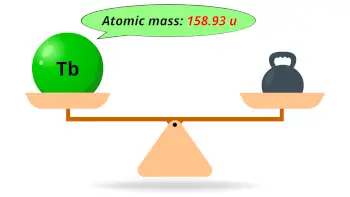 158.93 u |
| Electrons arrangement or Bohr model | 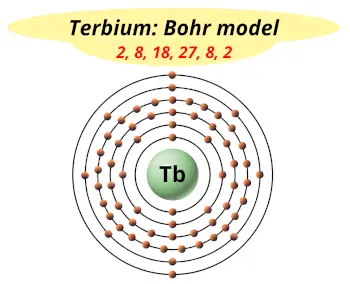 2, 8, 18, 27, 8, 2 |
| Electronic configuration | [Xe] 4f9 6s2 |
| Atomic radius | 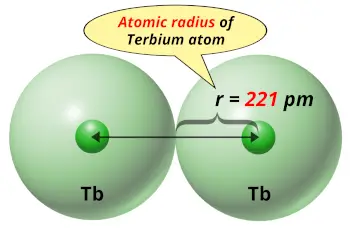 221 picometers (van der Waals radius) |
| 1st Ionization energy | 5.864 eV |
| Crystal structure | 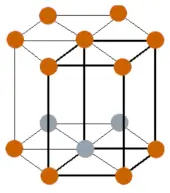 HCP (Hexagonal close packed) |
| Melting point | 1629 K or 1356 °C or 2473 °F |
| Boiling point | 3396 K or 3123 °C or 5653 °F |
| Density | 8.22 g/cm3 |
| Main isotope | 159Tb |
| Who discovered Terbium and when? |  Carl Gustaf Mosander in 1843 |
| CAS number | 7440-27-9 |
Terbium in Periodic table
Terbium element is in period 6 and in lanthanide group of the Periodic table. Terbium is the f-block element and it belongs to inner transition metals group.
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba | La* | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| *Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| **Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
←Move to: Gadolinium (Gd) element – Periodic Table
→Move to: Dysprosium (Dy) element – Periodic Table
Why is Terbium in Period 6?
Let me ask you a question.
How many shells does terbium have?
It’s 6. Right?
You have already seen the bohr model of terbium atom in the above table.
From the Bohr model, it can be found that the number of orbits or shells in terbium is 6. Hence, as terbium has 6 orbits, it lies in period 6 of the Periodic table.
Why is Terbium in f-block?
Before knowing this reason, first of all I want to ask you a simple question.
How can you determine the blocks-wise position of elements?
The simple answer: The elements will lie in the s, p, d or f block will completely depend upon the subshell in which the last electron will enter.
For example; the electron configuration of terbium is [Xe] 6s2 4f9.
So the last electron of terbium enters the f-subshell or f-orbital.
Hence, terbium is the f-block element.
5 Interesting facts about Terbium
Interesting facts about terbium element are mentioned below.
- The name terbium was derived from the name of a small village of Sweden (i.e “Ytterby”).
- Terbium was discovered by Carl Gustaf Mosander in 1843.
- The abundance of terbium in the earth’s crust is approximately 0.9 ppm by weight.
- The terbium element is considered as a rare earth metal, but it is not actually rare in quantity. The fact is that this metal is evenly distributed on the earth and hence it is difficult to find it from a single place. Hence it is rare in terms of available resources.
- “Monazite” is the most common mineral from which the terbium metal is commercially made. This is done by using a complex ion exchange process.
Properties of Terbium
The physical and chemical properties of terbium element are mentioned below.
Physical properties of Terbium
Physical properties of terbium are mentioned below.
- Terbium is a ductile as well as malleable metal that possess a silvery white metallic appearance.
- The terbium metal is soft and it can be cut even with a kitchen knife.
- The melting point and boiling point of ytterbium are 1356 °C and 3123 °C respectively.
- The atomic mass of terbium is 158.93 u and its density is 8.22 g/cm3.
- Terbium has many isotopes, but the most abundant isotope is 159Tb (which has an abundance of almost 100%).
- The crystal structure of terbium is HCP (Hexagonal close packed).
Chemical properties of Terbium
Chemical properties of terbium are mentioned below.
- Terbium is reactive metal and hence it is not found in its free state. But it is always found as a compound with other elements.
- During a chemical reaction, terbium generally forms 3+ ions. But it also forms 4+ ions very easily.
- The 3+ ions of terbium emit a green light when they are excited by appropriate wavelengths of light.
Uses of Terbium
Uses of terbium are mentioned below.
- Terbium glows green in color and it is used in euro notes.
- As terbium ions can emit green light, it is also used as a phosphor in color TV tubes.
- Terbium chloride, which is a compound containing terbium, is used to detect microbes.
- The alloy of terbium, neodymium and dysprosium are used in manufacturing magnets which can retain the magnetism even at higher temperature.
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
External resources:
- Terbium – Wikipedia. (2022, February 1). Terbium – Wikipedia. https://en.wikipedia.org/wiki/Terbium
- Terbium – Element information, properties and uses | Periodic Table. (n.d.). Terbium – Element Information, Properties and Uses | Periodic Table. https://www.rsc.org/periodic-table/element/65/terbium
- P. (n.d.). Terbium | Tb (Element) – PubChem. Terbium | Tb (Element) – PubChem. https://pubchem.ncbi.nlm.nih.gov/element/Terbium
- It’s Elemental – The Element Terbium. (n.d.). It’s Elemental – the Element Terbium. https://education.jlab.org/itselemental/ele065.html
- Atomic Data for Terbium (Tb). (n.d.). Atomic Data for Terbium (Tb). https://physics.nist.gov/PhysRefData/Handbook/Tables/terbiumtable1.htm
