Interactive Periodic Table of Elements
Jump to your interested topics from below.
#1 What is a classification? And why we need classification?
#2 Brief history of periodic table
#3 How are elements arranged in periodic table?
#4 Rows and columns in periodic table
#6 How to read periodic table elements?
#7 What does group number and period number represent on the periodic table?
#9 Trends in periodic table (Periodic trends)
What is a classification? And why we need classification?

What if someone asks you to find out one particular book from this huge stack of books.
Will you be able to find that particular book easily?
The answer is: NO

Look at this image, you can see that all the books are arranged in a particular shelf according to their similarities.
(In other words, books are grouped or sorted according to their similarities.)
Now, what if you have to find a particular book from this properly arranged shelf?

It becomes easy for you to find that particular book, right?
The same thing is with elements.
There are total 118 elements discovered till now.
These elements also need to be classified or grouped together according to their similarities.
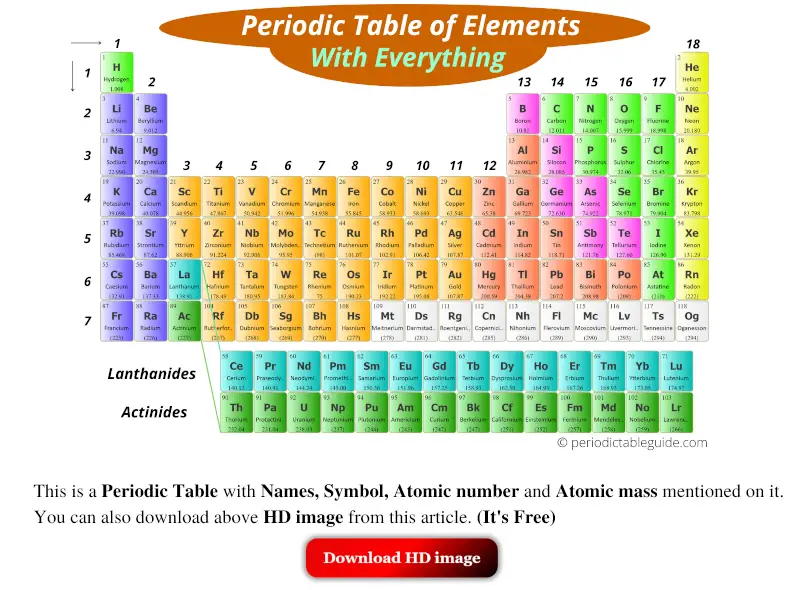
Now, this table looks amazing and well classified. Isn’t it?
Well, let me come to the main point.
Why classification of elements is needed?

A big question. Why do we need to classify all the elements?
All the elements have their own physical and chemical properties.
These elements have different uses, applications, chemical reactions, atomic structure, electron configuration, etc.
Thus it is necessary to classify (or group or sort) all these 118 elements according to their similarities.
After proper classification of elements, it becomes very easy for anyone to find a particular element from the table.
Also, the study of each and every element becomes easy by referring to the well classified elements.
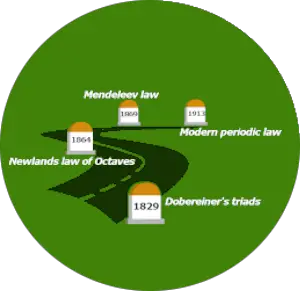
Brief history of periodic table
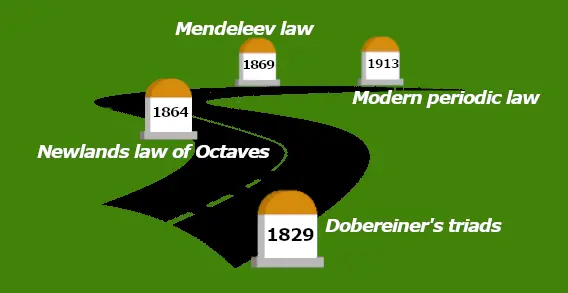
This picture shows you how the Periodic Table evolved.
History of Periodic table:
| Discovery | Year |
| Dobereiner’s triads | 1829 |
| Newlands law of octaves | 1864 |
| Mendeleev law | 1869 |
| The modern periodic law | 1913 |
Let me give you a brief introduction about the same.
1). Dobereiner’s triads (1829)
Johann Wolfgang Dobereiner (A person behind the discovery of triads)
But wait. What are triads?

Here is a meaning hidden in the name itself.
Tri means 3
During the time of Dobereiner, around 30+ elements were discovered.
Dobereiner arranged few of these known elements in the group of three elements.
Let me show you with an example.
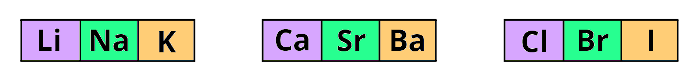
He arranged;
lithium (Li), sodium (Na) and potassium (K) in one triad (group of three elements),
Calcium (Ca), strontium (Sr) and Barium (Ba) in other triad and,
Chlorine (Cl), bromine (Br) and iodine (I) in other triad.
The main thing I want to tell you is that he arranged these elements in the increasing order of their ATOMIC MASS.
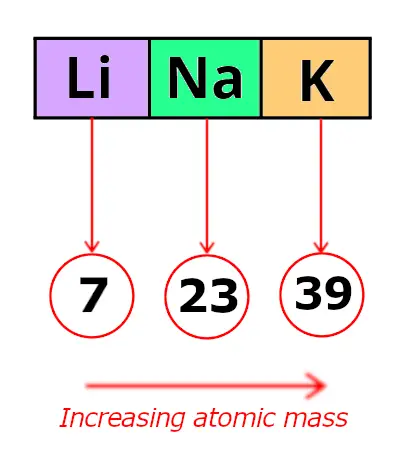
Now, let me recall to you the atomic mass of lithium, sodium and potassium.
Triad 1
| Element | Atomic mass |
| Lithium (Li) | 7 |
| Sodium (Na) | 23 |
| Potassium (K) | 39 |
In these triads, Dobereiner found that the atomic mass of the middle element is the average of the atomic mass of the first and third element.
I’ll explain.
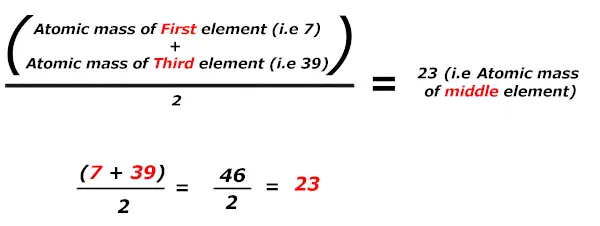
Kudos, big magic !!
Same way this similarity was seen for second triad as well as third triad.
Statement of Dobereiner’s triads:
When elements are arranged in increasing order of their atomic mass, in group of three (triads), then the arithmetic mean of mass of 1st and 3rd element is found to be approximately equal to the mass of central element.
He also stated that the elements of the same triad show similar properties. (Means Lithium, sodium and potassium are in same triad and so they shows similar properties)
Limitations of Dobereiner’s triads
- Dobereiner’s triads were applicable for only few elements. It was not applicable for all the 30+ known elements.
Hope you have understood this, now let’s move to the next discovery.
2). Newland’s law of octaves (1864)

A man behind the discovery of Newland’s law of octaves: John Newlands
A basic question. What is octave?

Oct indicates the 8
(Note: It is not October, but if you know about the IUPAC nomenclature, then you might have heard that oct is used to represent 8)
John Newlands discovered his law of octaves in the year 1864.
During this time, 56 elements were discovered.He arranged these known elements in the increasing order of their ATOMIC MASS.
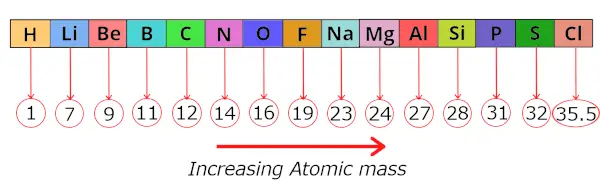
He found that after this arrangement, every 8th element shows a similar property as that of its respective 1st elements.
Let me give you an example.
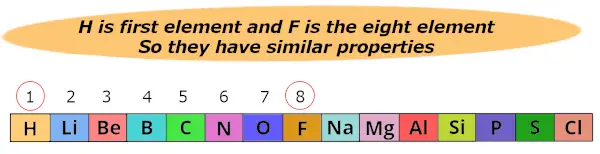
Here hydrogen (H) is 1st element and Fluorine (F) is the 8th element.
So Newlands found that these two elements show similar properties.
Another example;
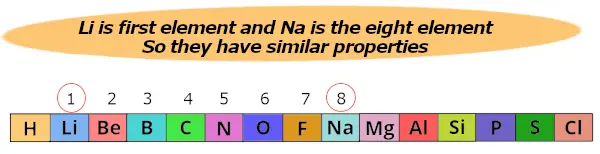
Here lithium (Li) is 1st element and Sodium (Na) is the 8th element.
So both these elements show similar properties.
Thus, Newlands found that if all the elements are arranged in increasing order of their atomic mass, then every eight elements shows the similar property.
Hence, the name of this law is Newland’s law of octaves.
Statement of Newland’s law:
If elements are arranged in the increasing order of their atomic mass, then the property of every eight elements (starting from the first element) repeats.
Limitations of Newlands law of octaves
- Similarity in properties as per the law was seen up to calcium only.
- He stated that only 56 elements exist in nature. But later on, he was proved wrong and many more elements were discovered.

- Dissimilar elements were placed in the same slot.
- Similar elements were placed in different slots.
3). Mendeleev law (1869)

Wow, what a personality !!!
Amazing.
Mendeleev law:
The properties of elements are the periodic functions of theirs atomic masses
Dmitri Ivanovich Mendeleev discovered this law and it is known as Mendeleev law.
Well, let me explain this to you with a simple story.
It’s a story of 1869.
During that time, 63 elements were known.
Mandeleev prepared the cards (just like the solitaire cards) of all these 63 elements with their individual properties written on each card.
He took a board and then he arranged all these 63 elements in the increasing order of their atomic mass.
First he took the element with lowest atomic mass, and started placing other elements besides it.
If the property of any of the elements were similar, then he used to place it in the same column. And if the property of elements did not match, then he kept it in the row.
That way he filled the entire table and finally the table was prepared as shown below.
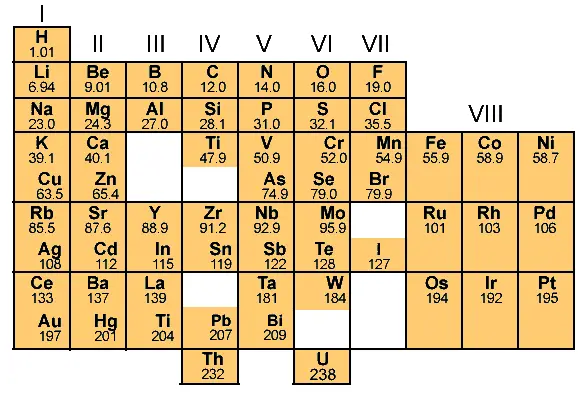
This table is known as the Mendeleev periodic table of elements.
There are a total 8 vertical columns in the Mendeleev periodic table, which are known as groups.
And there are 7 horizontal rows, which are known as periods.
But wait.
Have you noticed that many blocks have more than one element in Mendeleev periodic table? Why?
The short answer: Those elements show similarly in their properties. That’s why Mendeleev placed those elements in the same block.
One more question.
Why are some blocks empty in the above Mendeleev periodic table?
Now look, while arranging the elements according to atomic masses, Mendeleev found that the properties of few elements were not matching with any of the previous ones, so he didn’t place such elements in that particular column.
So there were some empty blocks seen on the Mendeleev periodic table.
Understood?
I know this seems a little difficult for you, so you can refer to this detailed guide on “Mendeleev periodic table” where I have explained all these things with proper images for your better understanding. I have also discussed the merits and demerits of the Mendeleev periodic table. So must visit that article.
4). Modern Periodic Law (1913)
We have seen in previous section that Dobereiner’s law, Newland’s law as well as Mendeleev law were based on the arrangements of elements according to their increasing atomic masses.
But the only difference in modern periodic law is;

Yes, all the elements in modern periodic table are arranged on the basis of their ATOMIC NUMBER.
Modern periodic law:
The properties of the elements are the Periodic function of their ATOMIC NUMBERS.
Modern Periodic Law was given by Henry Moseley in 1913.
This law is exactly similar to the Mendeleev law, but the only difference is;
- Mendeleev arranged the elements according to the increasing atomic mass. While,
- Henry Moseley arranged the elements according to the increasing atomic number.
In other words;
- Mandeleev law says that the properties of elements are the functions of their atomic mass. While,
- Modern periodic law says that the properties of elements are the Periodic function of their atomic number.
According to the modern periodic law given by Henry Moseley, the elements should be arranged in the table on the basis of their ATOMIC NUMBER.
That’s it!
After arranging all the elements based on atomic number, the modern periodic table came into existence.
How are elements arranged in periodic table?
Here, you will exactly come to know how Henry Moseley arranged the elements in periodic table on the basis of modern periodic law.
As said in his modern periodic law, the elements should be arranged according to the increasing atomic number.
So the minimum atomic number (i.e 1) was placed at the left-top corner of the table.
Further, the elements with atomic number 2, 3, 4, 5, 6, etc… were placed in the blocks of periodic table.
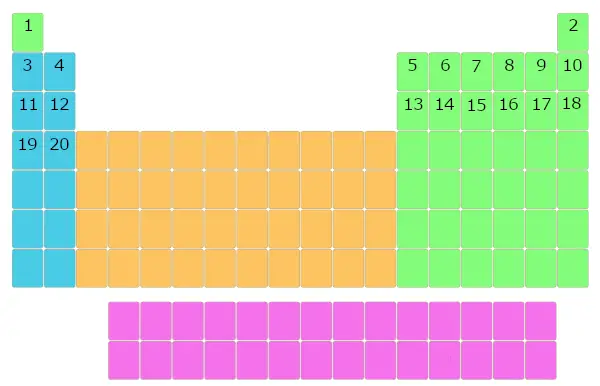
Similarly, all the elements were filled in this table of elements.
But, here is an important thing to know.
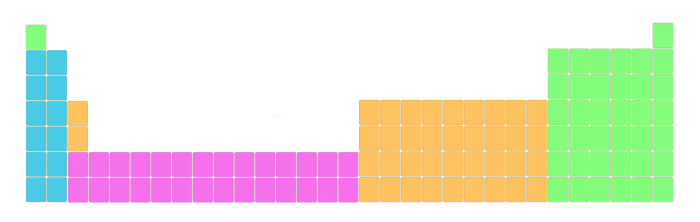
See the above image. The actual periodic table is like this. This Periodic table is also known as a long form of periodic table.
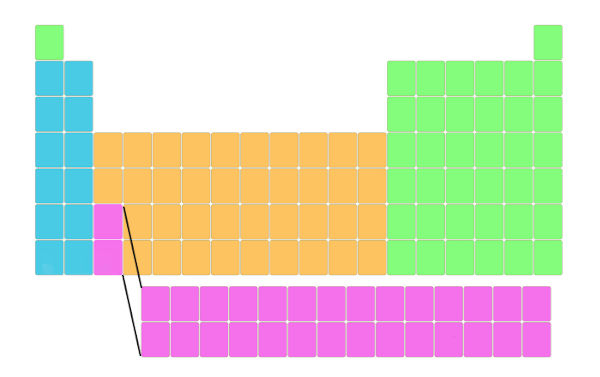
But it is represented like this so that it fits perfectly and it will not distort the shape of the periodic table. Also the elements lying in this block have different electron configuration and different properties. Well, do not worry about this, I’ll explain to you about the electronic configuration and properties later.
Still confused?
Look, the element having atomic number 57 is here as shown in the below picture.
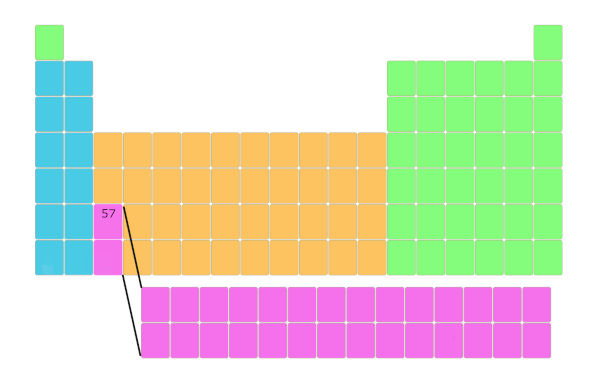
Now, element 58 is not placed besides it, but it is placed in a separate block as shown below. And this repeats up to element number 71.
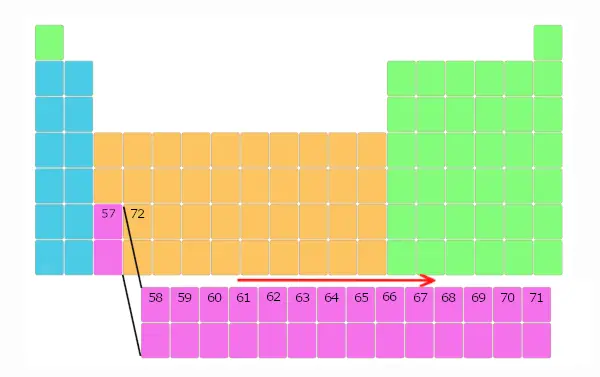
Thus, elements with atomic number 58 to 71 are placed in separate block below the main table.
Similarly, elements with atomic number 90 to 103 are also placed in separate block as shown below.
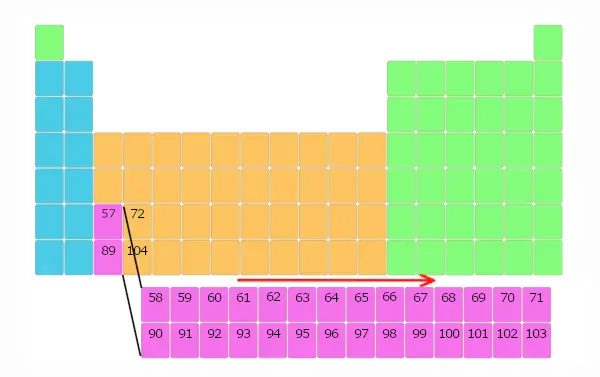
Just wait for few seconds, You will definitely come to know the reason why these elements are placed in a separate block.
Keep reading…
Rows and columns in periodic table
I want to explain you the basics of periodic table, but before that, let us proceed with the rows and columns of the Periodic table.
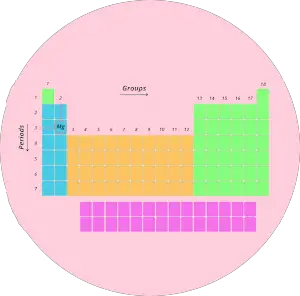
1). Rows in periodic table (Periods)
There are total 7 rows in periodic table.
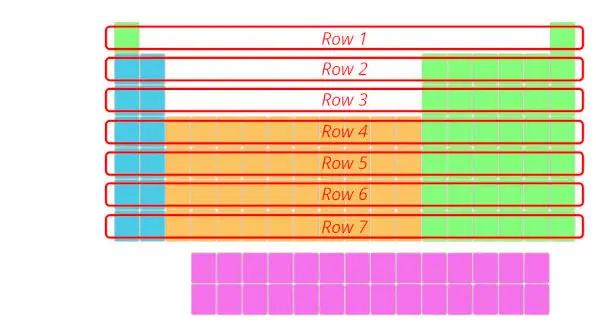
Question: You might be having a question that “What are Periods in Periodic table?”
Answer: The rows in Periodic table are known as Periods.
The first row is known as 1st period, second row is known as 2nd period and similarly you can see in the below image till the 7th row.
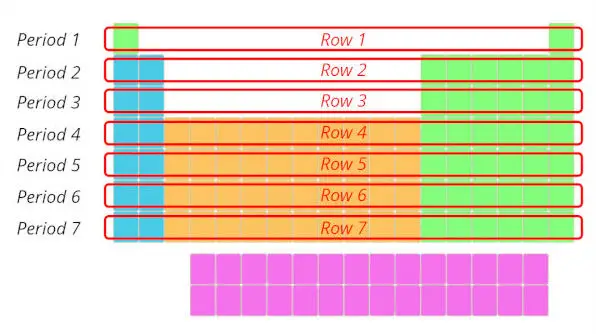
Now, let’s move to the columns of the Periodic table.
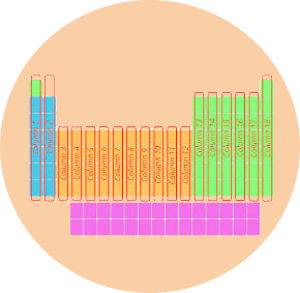
2). Columns in periodic table (Groups)
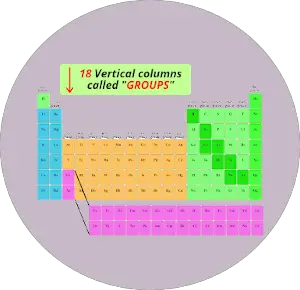
Just see below, there are total 18 columns in the Periodic table.
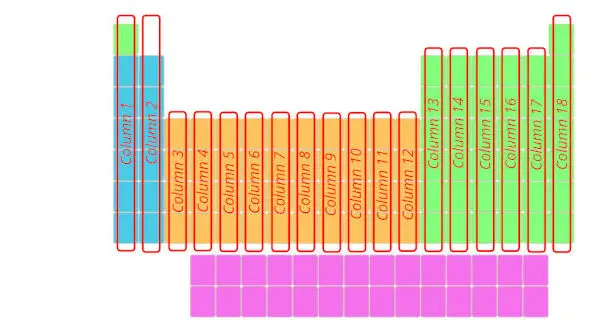
If you are having a question “What are Groups in Periodic table?”, then here is a short and sweet answer for you.
Answer: The columns in Periodic table are known as Groups.
The first column is known as the 1st group, second column is known as 2nd group and similarly you can see in the below image till the 18th column.
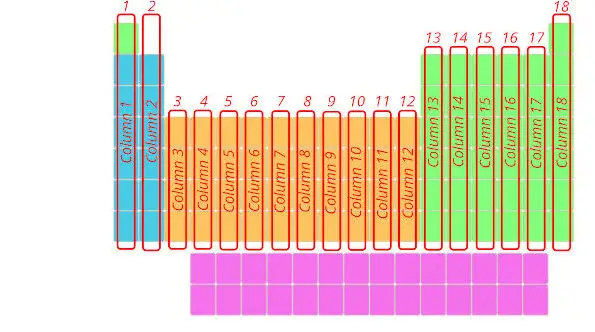
Now, it’s time to see the entire periodic table. Hurray!!!
Basics of periodic table
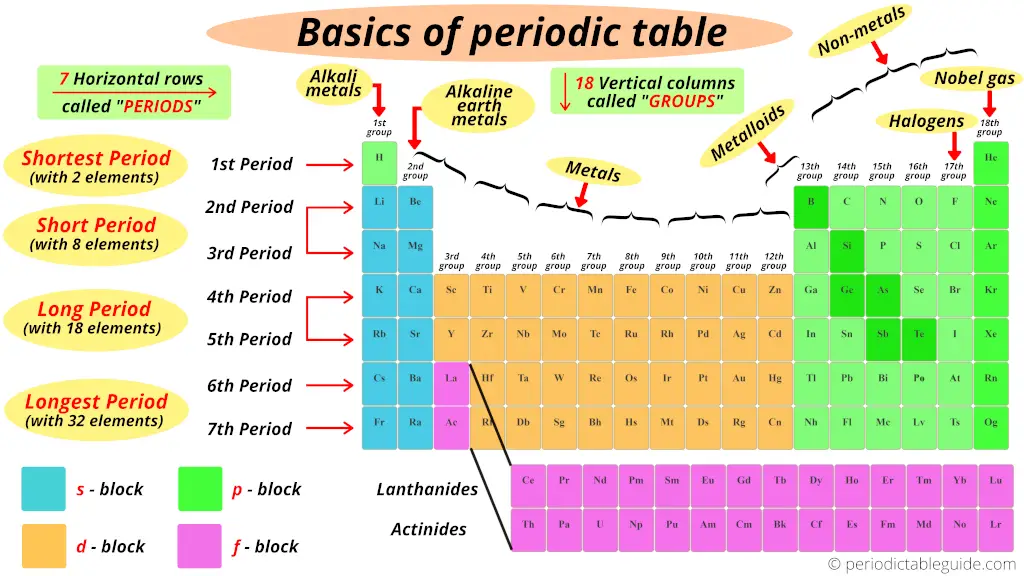
In this section you will come to know all the basis things related to the modern periodic table like;
- Groups and periods of periodic table
- Blocks of Periodic table
- Types of Elements on periodic table
- How to read the periodic table?, etc..
1). Groups and periods of periodic table
We have already seen in the above section that the vertical columns in the modern periodic table are known as groups.
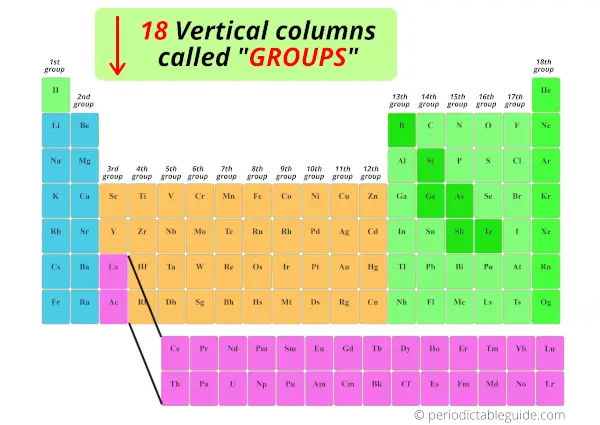
There are total 18 vertical columns in modern periodic table. (In other words, there are 18 groups in modern periodic table)
Now, I’ll tell you amazing things about Periods of the Periodic table.
We have seen that the horizontal rows of periodic table are known as periods.
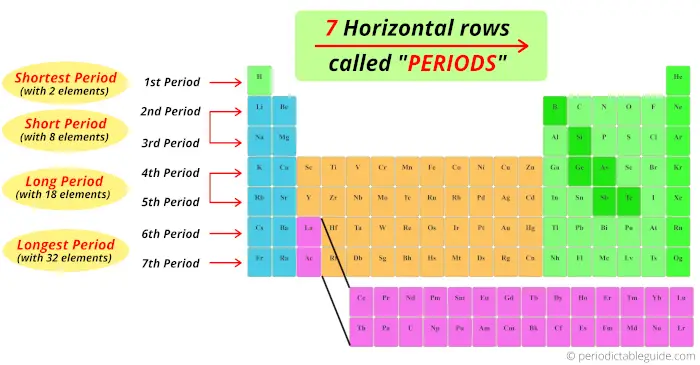
There are total 7 horizontal rows in periodic table. That means there are total 7 periods in periodic table.
Now, you can see that there are only 2 elements in the 1st period. Thus, period 1 is known as shortest period.
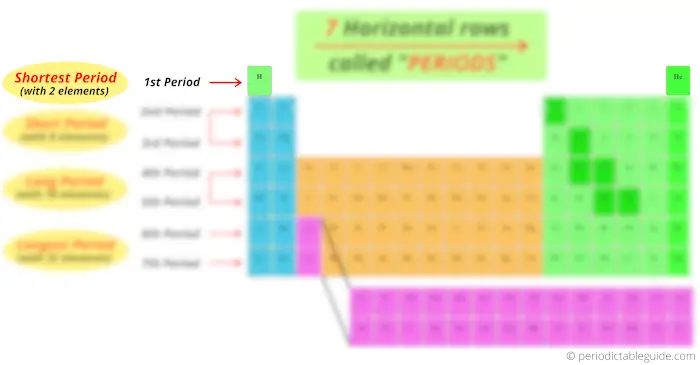
Now, the 2nd period and 3rd period has 8 elements, so period 2 and period 3 are known as short period.
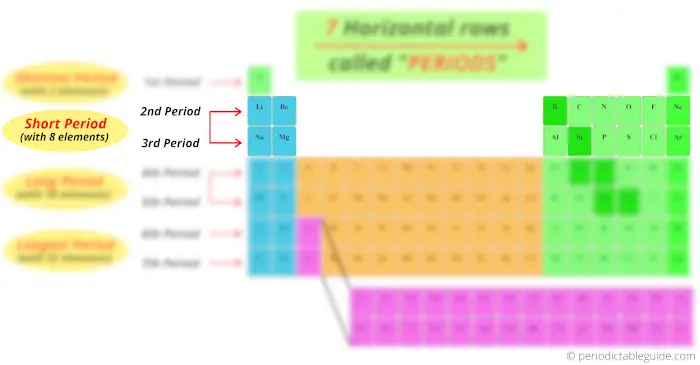
4th and 5th period has total 18 elements. So there are known as long period.
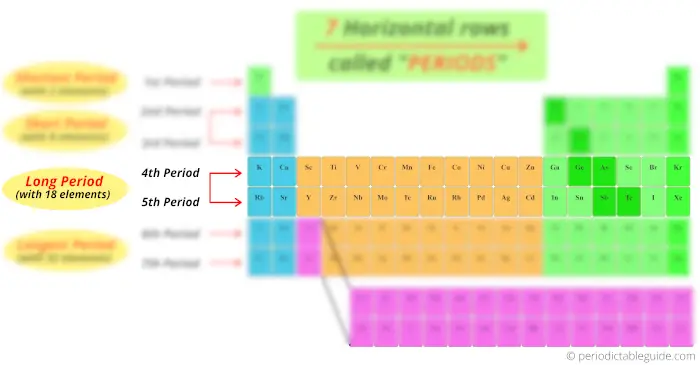
Now pay attention to this.
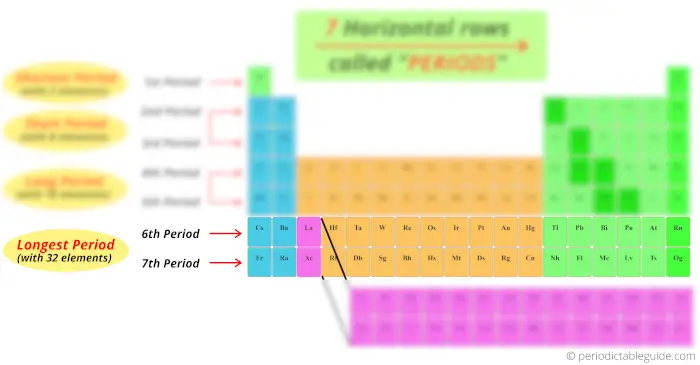
Look at the 6th and 7th period, how many elements are there?
There are 18 elements + 14 elements at the bottom block. So total 32 elements are present in period 6 and period 7.
Thus the 6th period and 7th period are known as longest period.
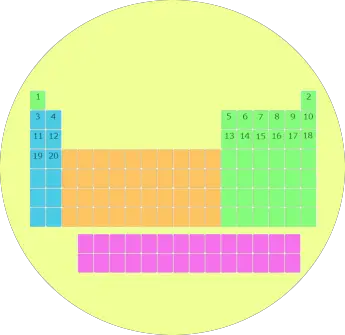
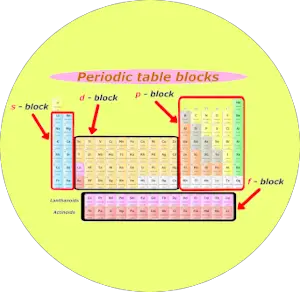
2). Blocks of periodic table
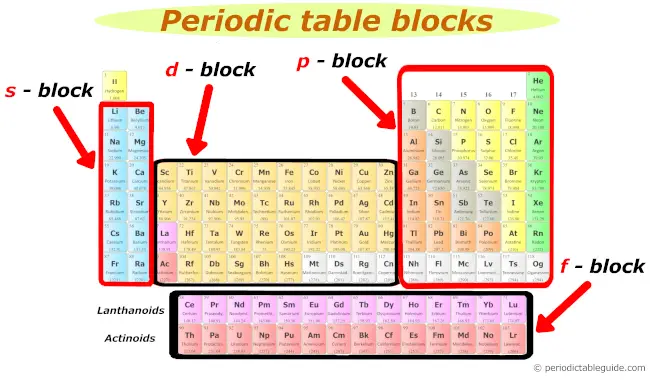
Well, these are the 4 blocks of periodic table.
Let me give you some overview of these 4 blocks one by one (in brief)
s block
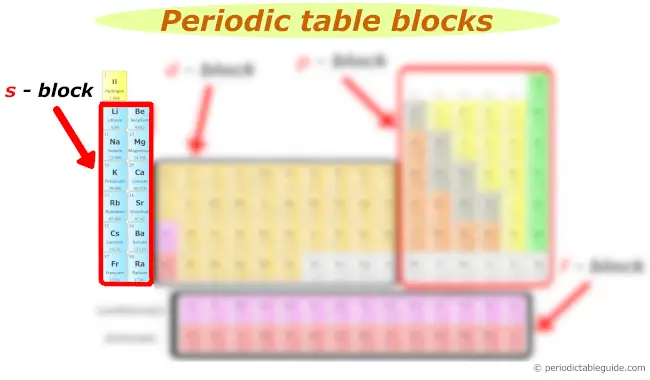
You can see the s block elements of the Periodic table in the above image.
s block includes the following groups:
- Group 1 and
- Group 2
List of group 1 elements with their symbol are given below:
| Element name | Symbol |
| Lithium | Li |
| Sodium | Na |
| Potassium | K |
| Rubidium | Rb |
| Cesium | Cs |
| Francium | Fr |
List of group 2 elements with theirs symbols are given below:
| Element name | Symbol |
| Beryllium | Be |
| Magnesium | Mg |
| Calcium | Ca |
| Strontium | Sr |
| Barium | Ba |
| Radium | Ra |
Also read: Why are these elements known as s-block elements?
p block
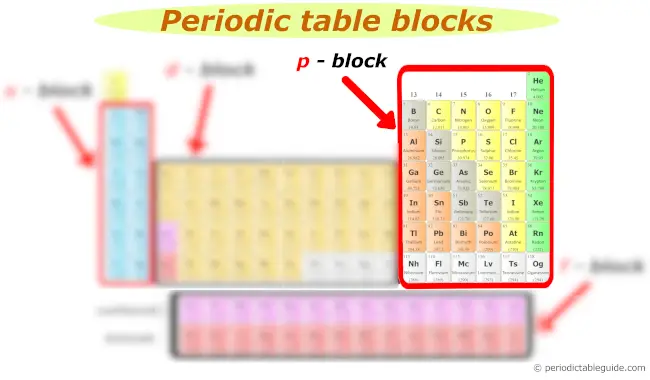
Now, here is your p block on the Periodic table.
The following groups are included in the p block of periodic table.
- Group 13
- Group 14
- Group 15
- Group 16
- Group 17
- Group 18
List of group 13 elements with their symbol are given below:
| Element name | Symbol |
| Boron | B |
| Aluminum | Al |
| Gallium | Ga |
| Indium | In |
| Thallium | Tl |
| Nihonium | Nh |
List of group 14 elements with their symbol are given below:
| Element name | Symbol |
| Carbon | C |
| Silicon | Si |
| Germanium | Ge |
| Tin | Sn |
| Led | Pb |
| Flerovium | Fl |
List of group 15 elements with their symbol are given below:
| Element name | Symbol |
| Nitrogen | N |
| Phosphorus | P |
| Arsenic | As |
| Antimony | Sb |
| Bismuth | Bi |
| Moscovium | Mc |
List of group 16 elements with their symbol are given below:
| Element name | Symbol |
| Oxygen | O |
| Sulphur | S |
| Selenium | Se |
| Tellurium | Te |
| Polonium | Po |
| Livermorium | Lv |
List of group 17 elements with their symbol are given below:
| Element name | Symbol |
| Fluorine | F |
| Chlorine | Cl |
| Bromine | Br |
| Iodine | I |
| Astatine | At |
| Tennessine | Ts |
List of group 18 elements with their symbol are given below:
| Element name | Symbol |
| Neon | Ne |
| Argon | Ar |
| Krypton | Kr |
| Xenon | Xe |
| Radon | Rn |
| Oganesson | Og |
Also read: Why are these elements known as p-block elements?
d block
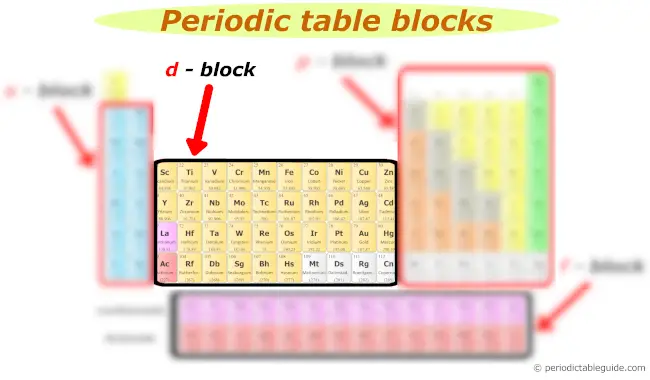
d block includes following groups.
- Group 3
- Group 4
- Group 5
- Group 6
- Group 7
- Group 8
- Group 9
- Group 10
- Group 11
- Group 12
The d block elements are also known as Transition elements.
List of d block elements with names is provided in the below table.
| 3rd Group | 4th Group | 5th Group | 6th Group | 7th Group | 8th Group | 9th Group | 10th Group | 11th Group | 12th Group |
| Scandium (Sc) | Titanium (Ti) | Vanadium (V) | Chromium (Cr) | Manganese (Mn) | Iron (Fe) | Cobalt (Co) | Nickel (Ni) | Copper (Cu) | Zinc (Zn) |
| Yttrium(Y) | Zirconium (Zr) | Niobium (Nb) | Molybdenum (Mo) | Technetium (Tc) | Ruthenium (Ru) | Rhodium (Rh) | Palladium (Pd) | Silver (Ag) | Cadmium (Cd) |
| Lanthanum (La) | Hafnium (Hf) | Tantalum (Ta) | Tungsten (W) | Rhenium (Re) | Osmium (Os) | Iridium (Ir) | Platinum (Pt) | Gold (Au) | Mercury (Hg) |
| Actinium (Ac) | Rutherfordium (Rf) | Dubnium (Db) | Seaborghium (Sg) | Bohrium (Bh) | Hassium (Hs) | Meitnerium (Mt) | Darmstadtium (Ds) | Roentgenium (Rg) | Copernicium (Cn) |
Also read: Why are these elements known as d-block elements?
f block
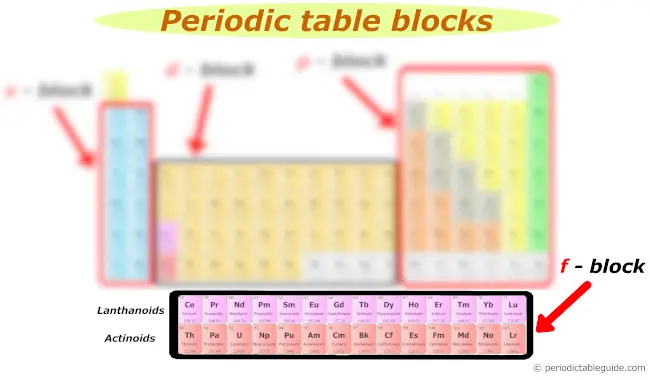
Many of you might have a question;
“What are the two rows at the bottom of the periodic table?”
They are known as Lanthanides and Actinides.
The first row of elements in the f block is known as Lanthanides and the second row of elements in the f block is known as Actinides.
The f block elements are also known as inner transition elements.
Also read: Why are these elements known as f-block elements?
3). Types of elements on periodic table
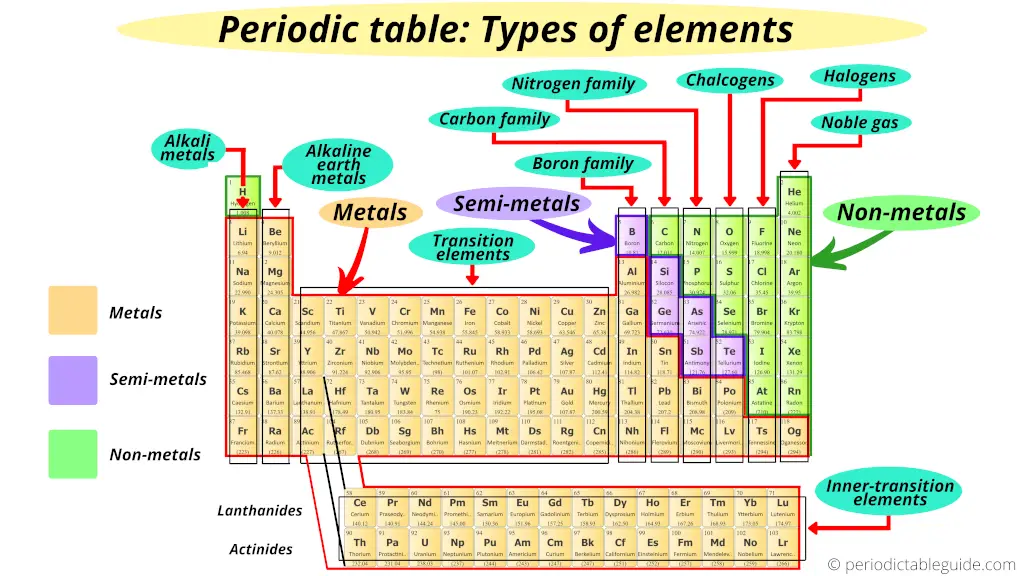
Now, I’ll show you what type of elements are there on the Periodic table.
You might have a question;
“Where are metals on the Periodic table?“
“Where are nonmetals located on the Periodic table?“
“Where are metalloids located on the Periodic table?“
“Where are halogens on the Periodic table?“
“Where are Noble gases on periodic table?“
And many more…
Where are metals on the Periodic table?
Look, first of all you should know some properties of metal, nonmetal and semimetal (metalloid).
But you will understand this position of elements even if you do not know anything. I’ll try to explain this in a simple way.
Just remember that, on the left side of the Periodic table there are the best metals.
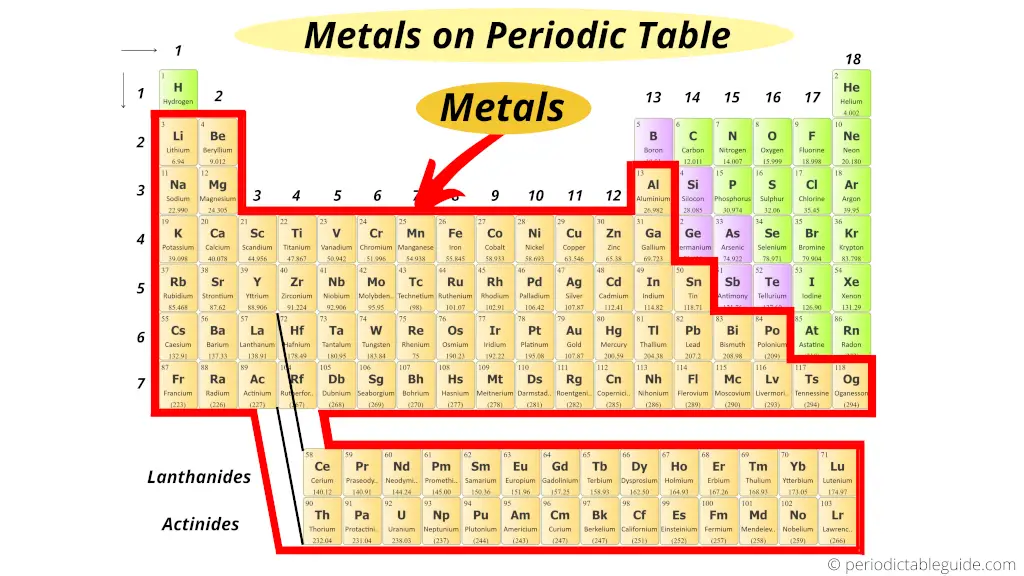
Further, according to their availability, they are again classified as Alkali metals and Alkaline Earth metals.
Also see:
1). How many metals are on periodic table?
2). Why are metals on the left side of periodic table?
Alkali metals:
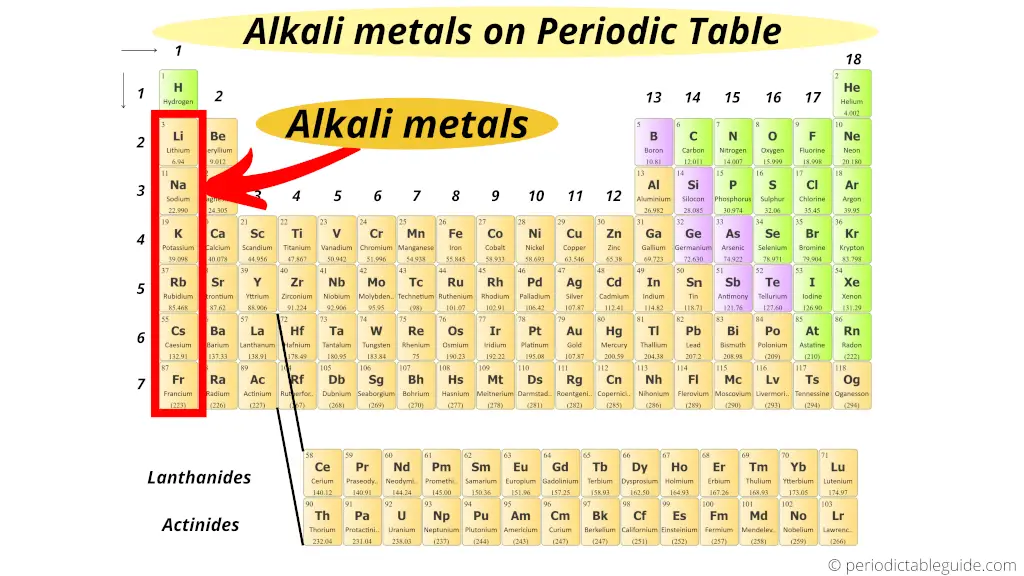
Group 1 elements are known as alkali metals.
All the elements of group 1 form an alkaline solution (or basic solution) when they react with water. (In other words, when they react with water, they form the compounds which are basic in nature)
That’s why these group 1 elements are known as alkali metals.
Also see:
1). Why are alkali metals so reactive?
2). Electron configuration of alkali metals
Alkaline Earth metals:
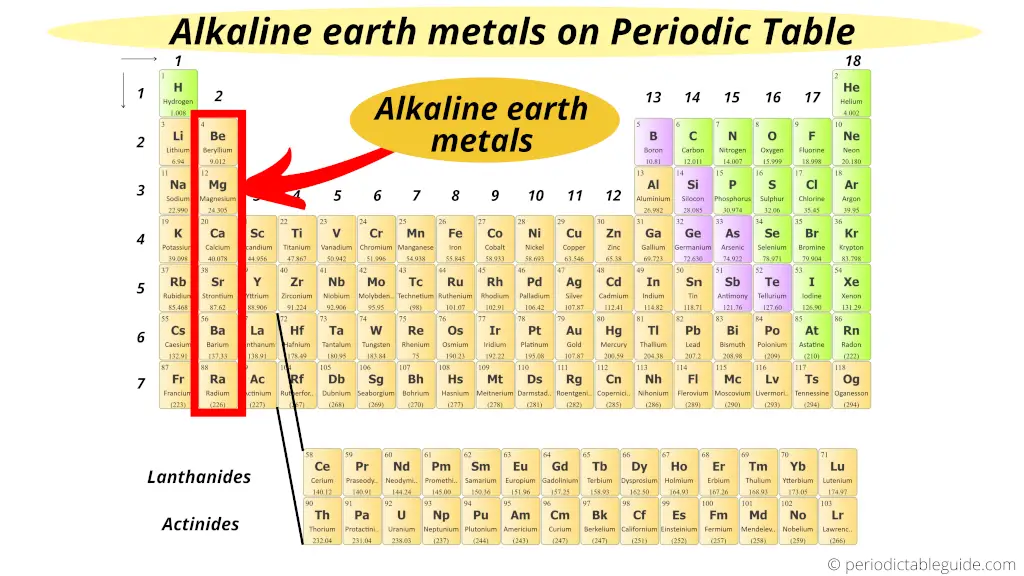
Group 2 elements are known as Alkaline Earth metals.
All these elements of group 2 have the similar properties as that of group 1 elements, but these elements are mostly found from the Earth crust. Hence, they are named Alkaline Earth metals.
Also see:
1). What do alkaline earth metals have in common?
2). List of alkaline earth metals with electron configuration
Where are nonmetals on the Periodic table?
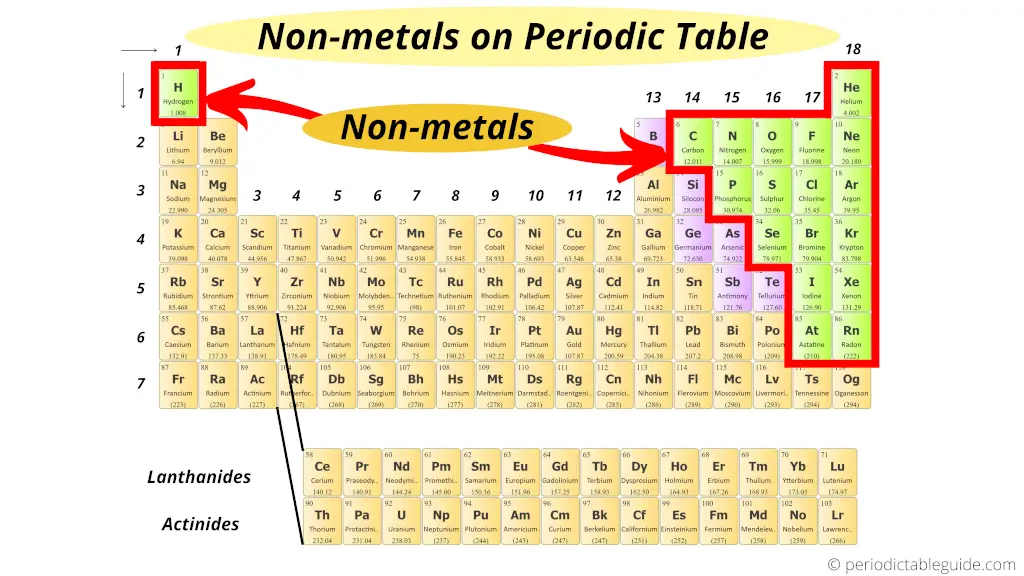
Here are the nonmetals on the Periodic table.
Read more about Nonmetals from here.
Where are metalloids (semimetals) on the Periodic table?
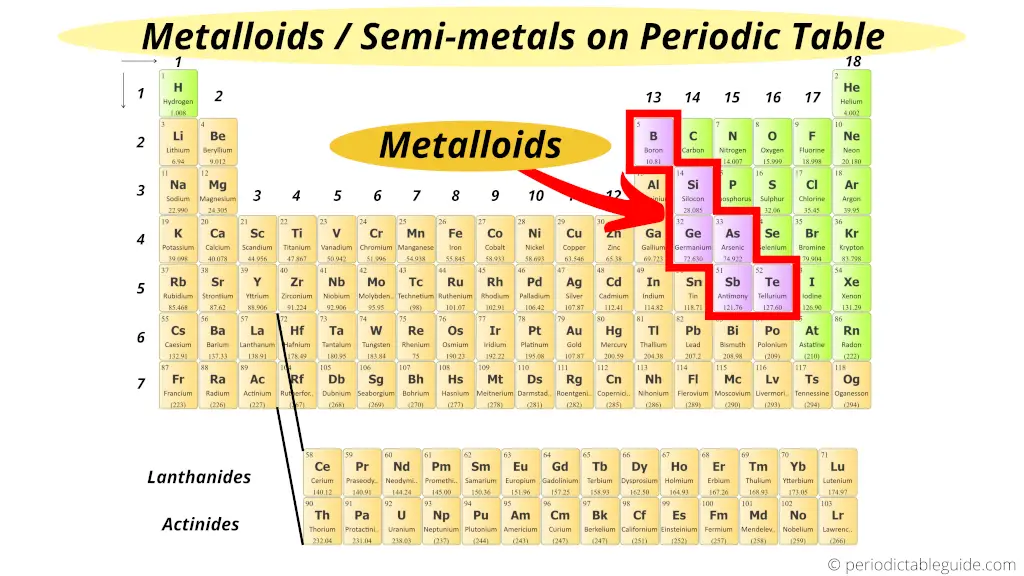
Here are the semimetals or metalloids on the Periodic table.
The zig zag line shown in the Periodic table includes the elements which possess metallic nature as well as nonmetallic nature.
Thus they are known as semimetals or metalloids.
Also see: Physical and chemical properties of metalloids.
Where are halogens on the Periodic table?
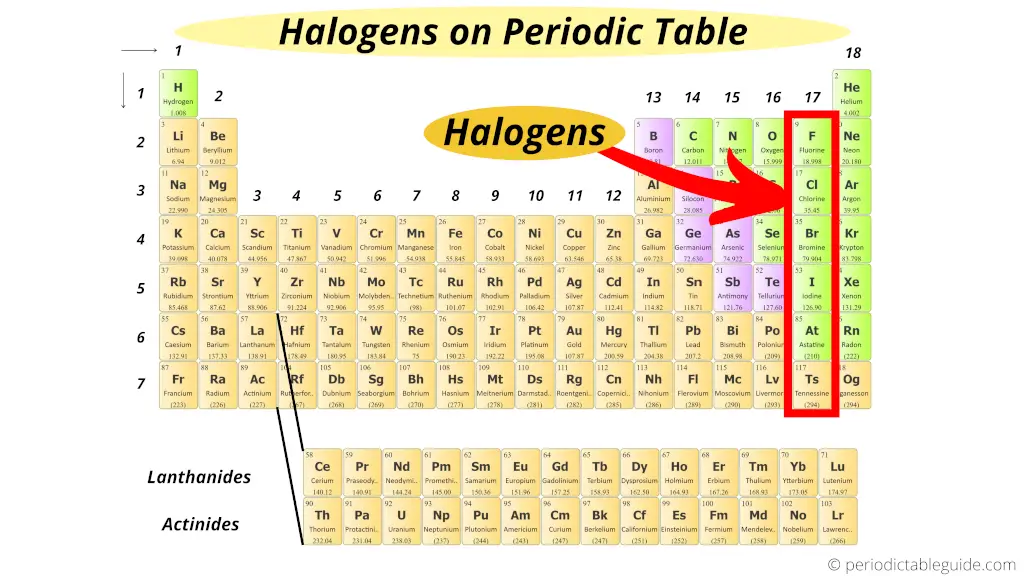
Do you know why halogens are called so?
The word Halogens has come from two Greek words;
“Hal” meaning “salts” and
“Gen” meaning “to produce”.
Thus “Halogens” means “salt producing”.
So all the elements of the group 17 form salts when they react with metals.
For example;
2Na + Cl2 ——-> 2NaCl
Which is a salt.
Thus, group 17 elements are known as Halogens.
Also see:
1). Which is most reactive halogen?
2). Properties of halogens.
Where are Noble gases on periodic table?
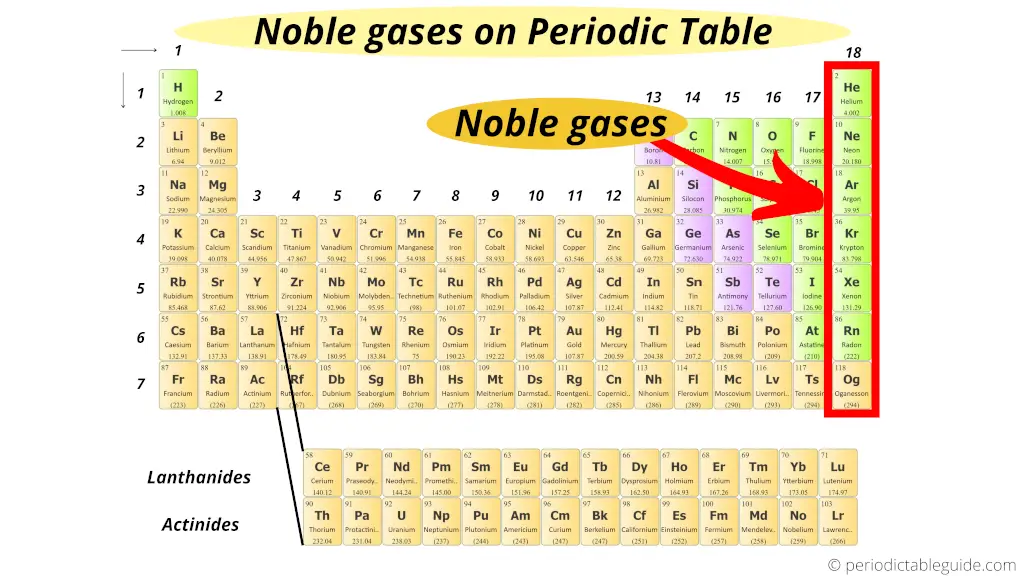
The elements of group 18 do not participate in any chemical reaction. (In other words, these elements are chemically inert)
Plus all the elements of group 18 are in gaseous state.
Thus, the 18th group elements of periodic table are known as Noble gases or inert gases.
Also see:
1). Electron configuration of noble gases
2). Uses of noble gases in daily life
Where are transition elements on periodic table?
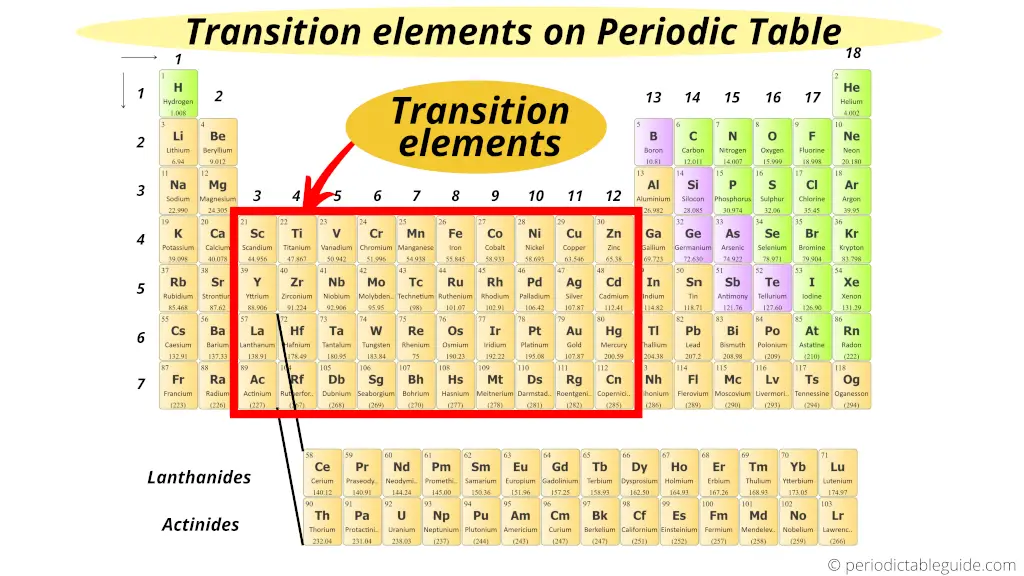
Transition metals (also known as Transition metals) form a bridge between the chemically active metals of s-block elements and the less active elements of Groups 13 and 14.
Thus these d block elements are known as “Transition Elements”.
Also see: List + Electron configuration of transition metals.
Where are inner transition elements on periodic table?
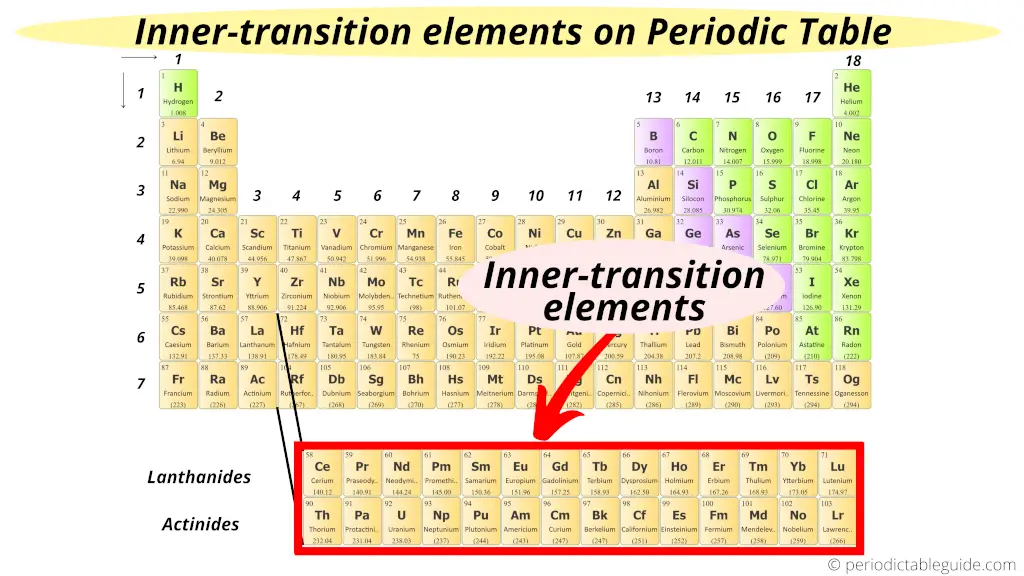
Now, this is very simple.
As the name suggest “inner”. Means these elements are transition elements only but they are placed in the inner section of transition elements.
Hence these f block elements are known as “Inner transition elements.”
Also see: Why are inner transition metals at the bottom of periodic table?
Where is boron family in the periodic table?
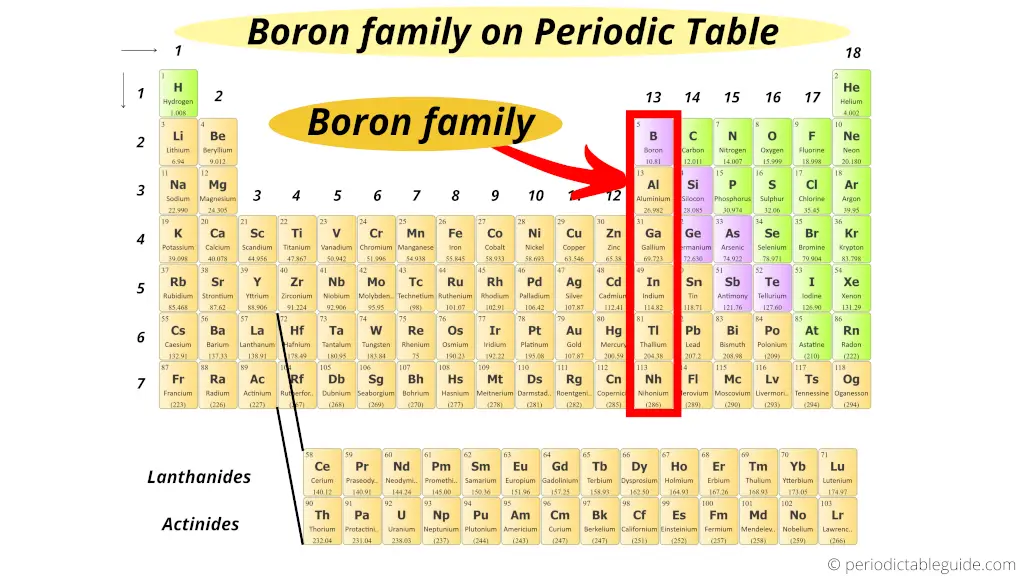
Very simple!!
The first element of group 13 is boron.
Hence, group 13 is known as boron family or boron group.
Where is carbon group in periodic table?
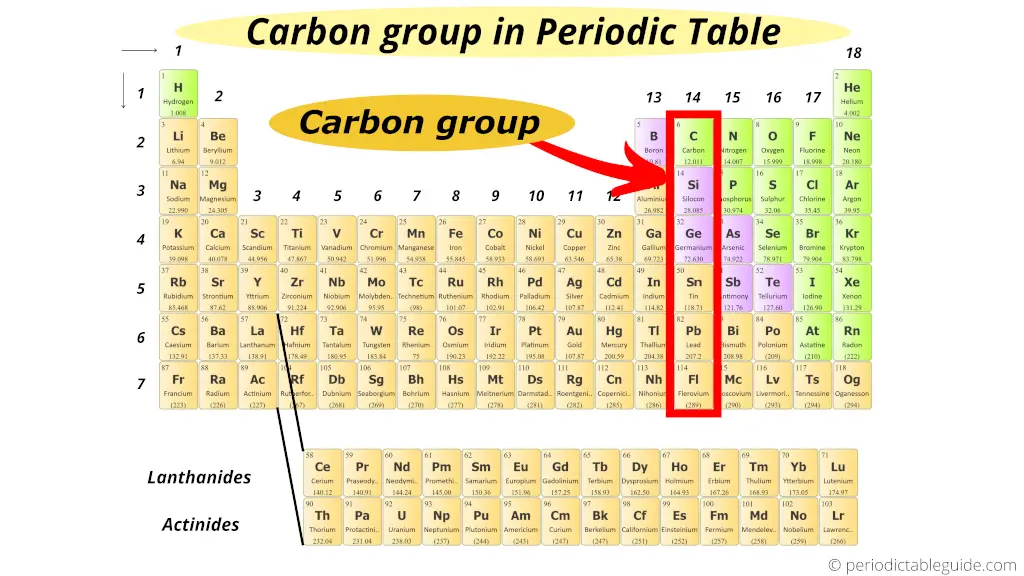
This is also simple!!!
The first element of group 14 is carbon.
Hence, group 14 is known as carbon family or carbon group.
Where is nitrogen family on periodic table?
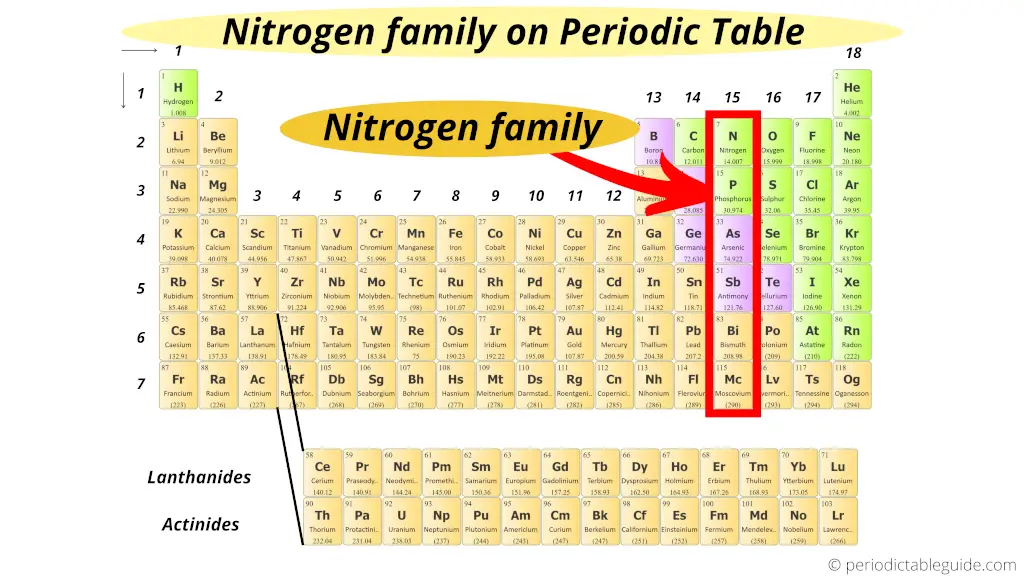
Group 15 elements has the first element “Nitrogen”.
So the group 15 is known as nitrogen family or nitrogen group.
These 15 group elements are also known as Pnictogens.
Where are chalcogens on periodic table?
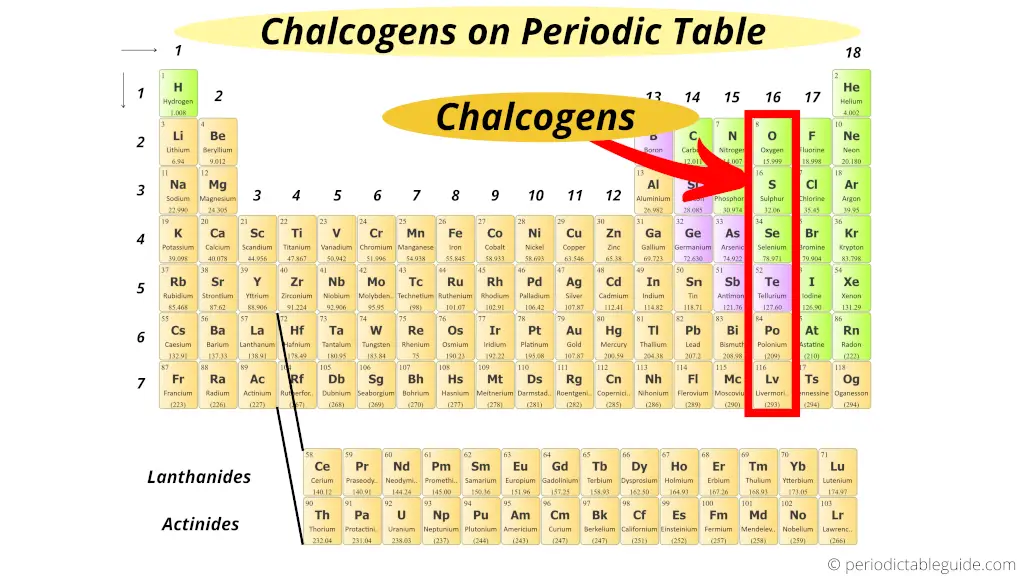
Group 16 elements includes oxygen, sulphur, selenium, tellurium and polonium.
These elements are generally present in the Earth crust in the form of ores.
Chalcogens is the Greek word which means “ore forming”.
As these group 16 elements are present in the ores, they are known as “Chalcogens.”
Enjoying?
If no, then also
Keep reading…. Because the most important part is coming now.
How to read periodic table elements?
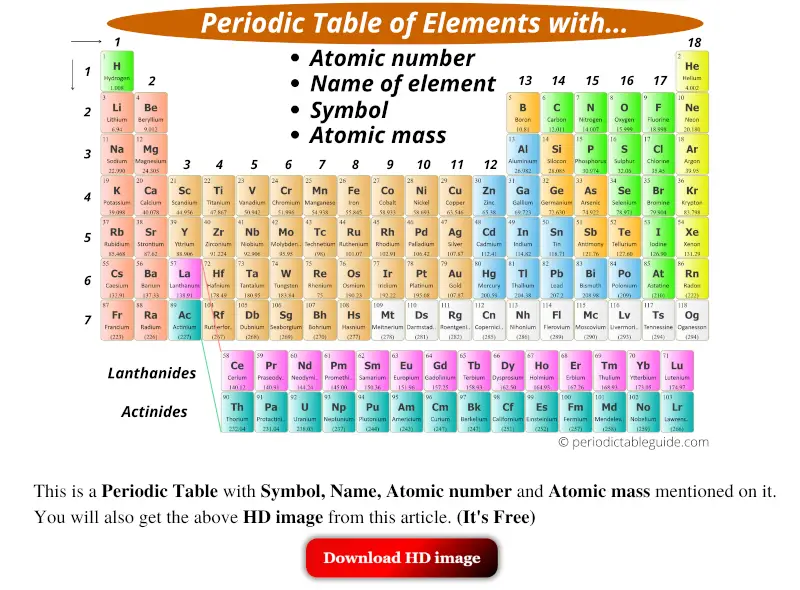
Here is your modern periodic table.
Now, you might be wondering;
- What is written in each single square box?
- What are those 1, 2, 3, 4, etc… numbers?
- What is that number written below each element?
- Why do the columns have different colors?
- What are those two rows at the bottom of the periodic table?
- How to remember periodic table?
- And many more…
Do not worry at all, I will explain everything step by step.
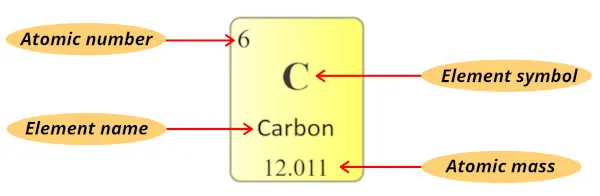
I will give you a very short and simple explanation for reading the elements of periodic table using the example of carbon element.
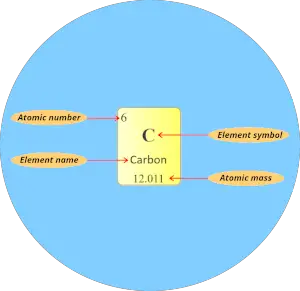
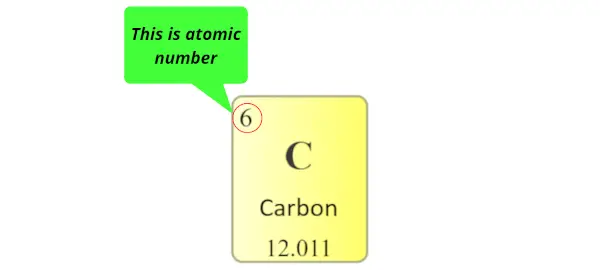
Atomic number:
The total number of protons present in the nucleus of an atom is known as Atomic number. Or
The number of electrons possessed by a neutral atom is called Atomic number.
Here, the number written on the top of each square is the atomic number of that element.
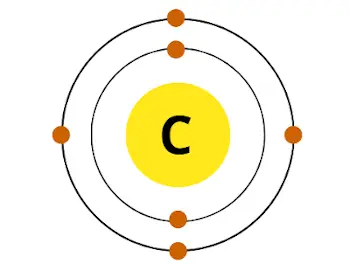
For example carbon has total 6 electrons. So the atomic number of carbon is 6.
Element symbol:
The alphabet used to mention the elements is known as the symbol of elements.
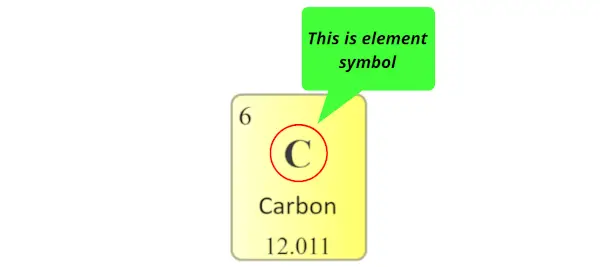
The symbol of elements are represented by using single uppercase alphabet (for e.g. C, O, N, F, H, etc…) or sometimes first letter is uppercase followed by lowercase alphabet (for e.g. Li, Na, Si, Ne, Ar, etc…)
Element name:
The name displayed in each individual square block represents the name of that element.
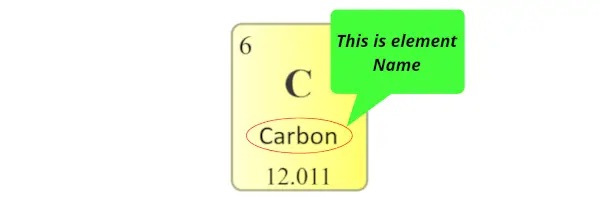
Atomic mass:
Atomic mass is the sum of the masses of protons and neutrons present in the nucleus of an atom.
Atomic mass in periodic table is displayed as shown in below image. (It is generally a decimal value)
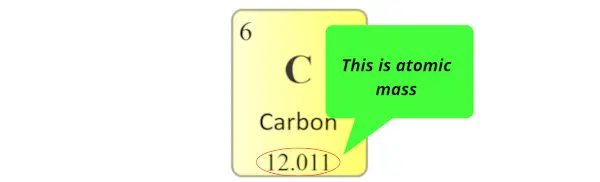
(Note: In some periodic tables, you may find that the atomic masses are written in the left top corner or right top corner of each cell, but remember that it is a decimal value. So if you find that decimal value in the cells of periodic table, it’s an atomic mass of that element)
Now the most important topic is coming…
If you are still with me, then You are going to Rock now.
Keep reading…
What does group number and period number represent on the periodic table?

Trust me this is very simple.
Let me explain directly with an example.
Example 1:
Let’s take an example of magnesium (Mg)
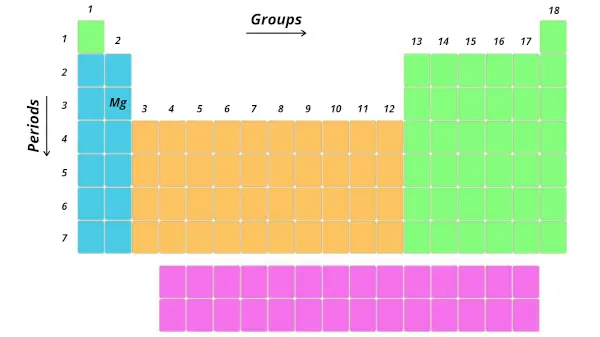
Now, tell me where is the position of magnesium (Mg).
It is in 3rd period and 2nd group. Right?
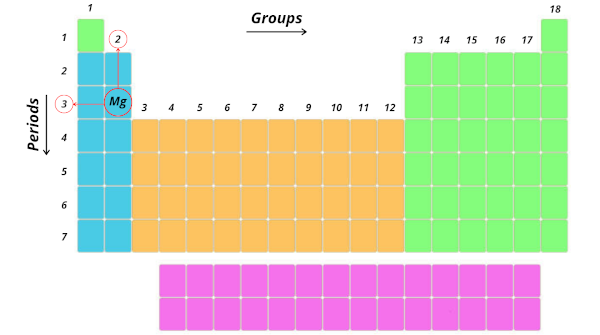
Cool…Now, this period 3 indicates that the atoms of magnesium possess 3 orbits or 3 shells.
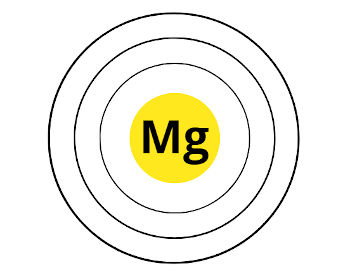
Here are the 3 orbits of magnesium.
Now, Magnesium (Mg) is lying in group 2. That means, it has 2 electrons in its outermost orbit.
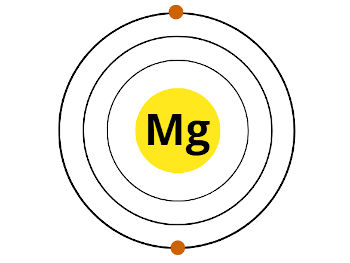
Here are the 2 electrons in the outermost orbit of magnesium. These two electrons are known as valence electrons.
And you might be knowing that each orbit has some different capacity to hold electrons.
See the table below, you will come to know “how many electrons a particular orbit can hold.”
Number of electrons in shells:
| Orbit / Shell (n) | Maximum no. of electrons this orbit can hold |
| K shell, n = 1 | 2 × 1² = 2 |
| L shell, n = 2 | 2 × 2² = 8 |
| M shell, n = 3 | 2 × 3² = 18 |
| N shell, n = 4 | 2 × 4² = 32 |
Thus,
- 1st shell can hold 2 electrons.
- 2nd shell can hold 8 electrons.
- 3rd shell can hold 18 electrons.
- 4th shell can hold 32 electrons.
Let’s come back to magnesium. It is having atomic number 12. That means it has total 12 electrons.
From the above table, we can say that, first orbit will hold 2 electrons, the second orbit will hold 8 electrons and finally remaining 2 electrons will be in the 3rd orbit.
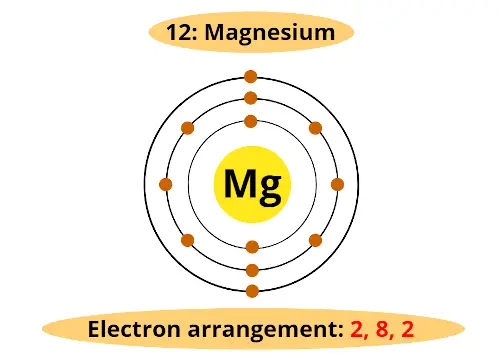
(Side note: The actual orbits are not 2 dimensional, but they are 3 dimensional. In order to represent on the paper, we draw 2 dimensional orbit)
Example 2:
Let’s take another example of Chlorine (Cl).
Chlorine lies in the 3rd period and 17th group.
3rd period indicates that chlorine has 3 orbits and;
17th group indicates that it has 7 electrons in outermost orbit.
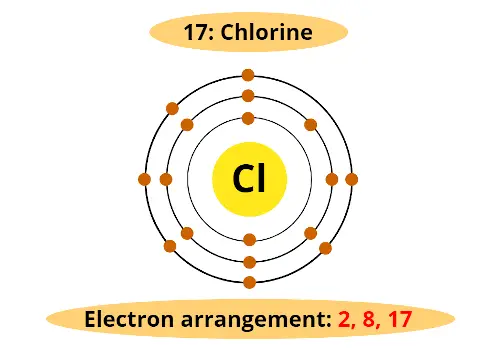
Atomic number of chlorine is 17. (In other words, Chlorine has total 17 electrons).
And now you know very well that 2nd orbit holds maximum 8 electrons.
Thus, the arrangement of electrons in the orbit of Chlorine atom is as shown above.
Remember:
Group 13 indicates 3 valence electrons, not 13.
Group 14 indicates 4 valence electrons, not 14.
Group 15 indicates 5 valence electrons, not 15.
Group 16 indicates 6 valence electrons, not 16.
Group 17 indicates 7 valence electrons, not 17.
Group 18 indicates 8 valence electrons, not 18.
I have explained the concept of valence electrons for d block and f block elements in separate articles “d block elements” and “f block elements.” (Because it is little bit different and first you need to know about the concept of orbitals)
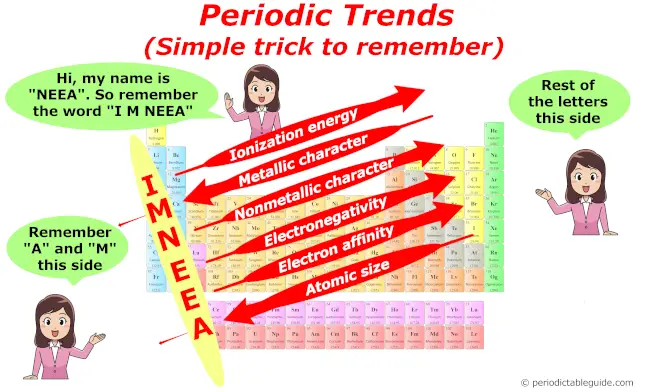
Trends in periodic table (Periodic trends)
I’ll keep this very short and to the point. (Because this article is very long now). If you want detailed information on this topic, you must visit: Main article on Periodic trends.
Let’s dive straight into it.
First of all, what does trends mean in Periodic table?
Trends: The change in properties of elements down the groups (from top to bottom) and across the periods (from left to right) is known as trends.
We generally measure these trends (change in properties of elements) down the group and across the period.
I’ll shortly tell you the variation of following properties in the periodic table.
- Valency trend
- Atomic size trend
- Metallic character trend
- Non metallic character trend
- Electronegativity trend
- Electron affinity trend
- Ionization energy trend
Periodic trends: Valency
As we move across the period (from left to right), the Valency of the elements first increases and then decreases. While moving down the group (from top to bottom), the Valency of elements remains the same.
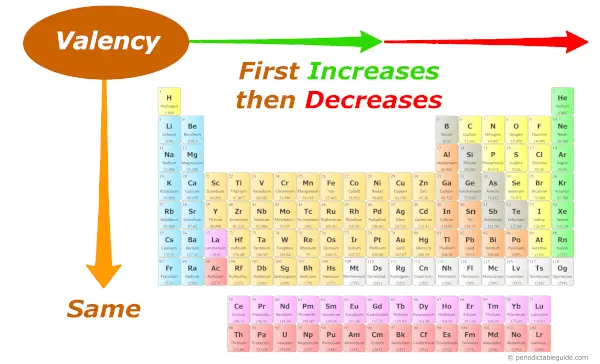
Don’t worry, I’ll tell you the reason why this happens.
See, first of all valency is nothing but the number of electrons required to complete the octet.
For example, atomic number of oxygen is 8. That means oxygen has total 8 electrons.
So it’s electron arrangement is (2, 6).
It has 2 electrons in first orbit and other 6 electrons in second orbit.
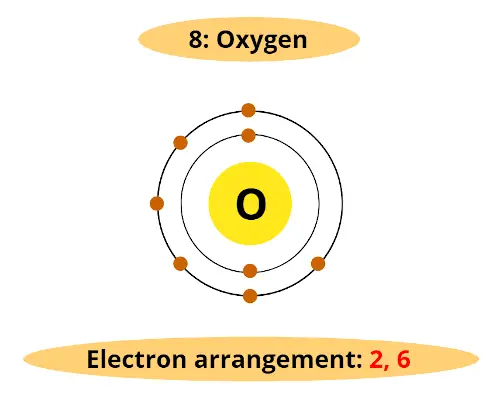
You can see that oxygen has 6 electrons in its outermost orbit.
Thus, it requires 2 electrons to complete the octet.
Hence valency of oxygen is 2.
As we move across the period (from left to right), the Valency of elements first increases and then decreases.
And along the group, the Valency remains constant, because you know very well that the number of electrons in the outermost orbit remains the same for all the elements of the same group.
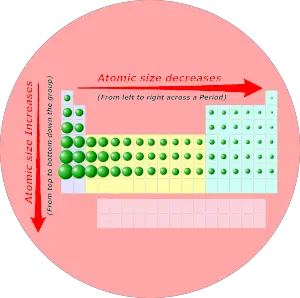
Periodic trends: Atomic size
As we move across the period (from left to right), the atomic size of elements decreases. While moving down in the group (from top to bottom), the atomic size increases.
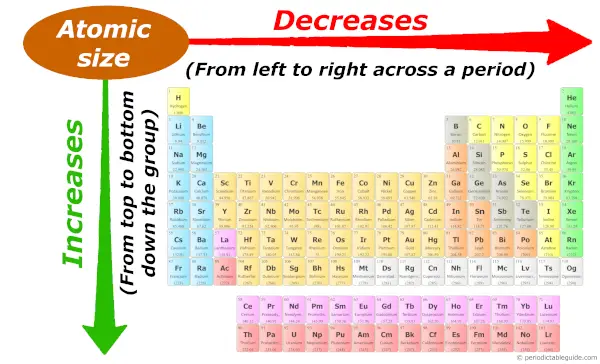
Want to know why the atomic size decreases across a period and why it increases down the group?
Then check out the separate article on Atomic size trends. (Where I have explained the reason with images and example).
Periodic trends: Metallic character
As we move across the period (from left to right), the metallic character of elements decreases. While moving down in the group (from top to bottom), the metallic character increases.
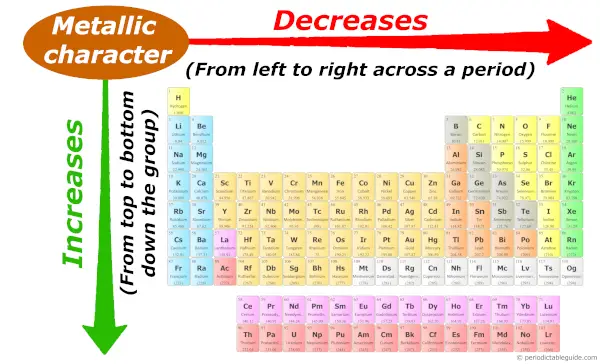
To know the reason behind this trend, I highly recommend you to visit the separate article: Metallic character trend in Periodic table.
Periodic trends: Non metallic character
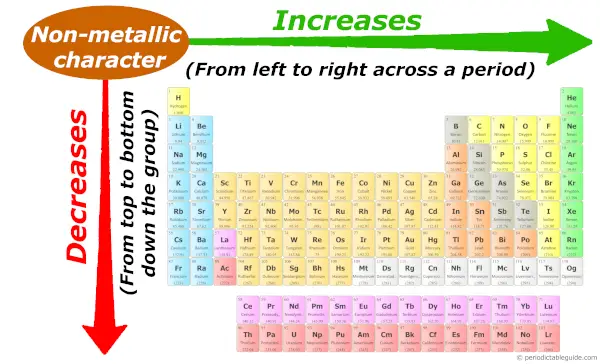
As we move across the period (from left to right), the non metallic character of elements increases. While moving down in the group (from top to bottom), the non metallic character decreases.
I have given little more information on non metallic character trends. So if you want to see it, then kindly visit this article: Non metallic character trend in Periodic table.
Periodic trends: Electronegativity
Down the group (from top to bottom), the atomic size increases. So Electronegativity decreases.
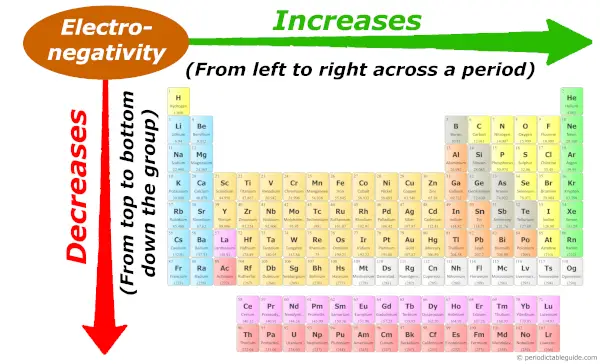
And across the period (from left to right), the atomic size decreases, so the Electronegativity increases.
But if you don’t know what electronegativity is, then you can refer to this article (where I have explained the simple plain english explanation about electronegativity and few more things about the electronegativity trend in Periodic table.)
Periodic trends: Electron affinity
As we move across the period (from left to right), the atomic size decreases, so electron affinity increases across the period (from left to right.)
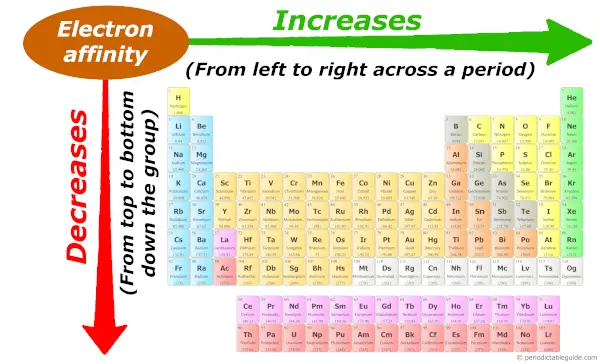
And as we move down the group (from top to bottom), the atomic size increases, so the electron affinity decreases down the group (from top to bottom). Now, if you don’t know about the concept of electron affinity, plus if you want to know the reason why electron affinity increases across a period and why it decreases down the group, then visit this article: Electron affinity trend in Periodic table.
Periodic trends: Ionization energy
Across the period (from left to right), the atomic size decreases, so ionization energy increases across the period (from left to right.)
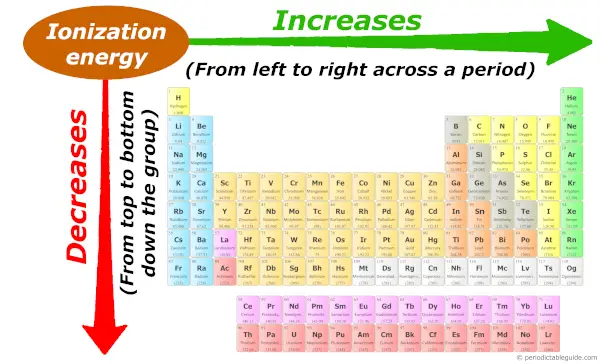
And down the group (from top to bottom), the atomic size increases, so the ionization energy decreases down the group (from top to bottom.)
Want to know why this trend is so?, then visit the Guide on Ionization energy trend, where I have explained the concept of ionization energy with images + I have also explained the reason why this trend is so?
Summary of Periodic trends
So as a summary, you can remember all the trends in periodic table with this single image.