This is a SUPER easy guide on Samarium element.
In fact, the table mentioned below is the perfect information box (Which gives you every single detail about the Samarium element in Periodic table.)
So if you want to know anything about Samarium element, then this guide is for you.
Let’s finish this very quickly.
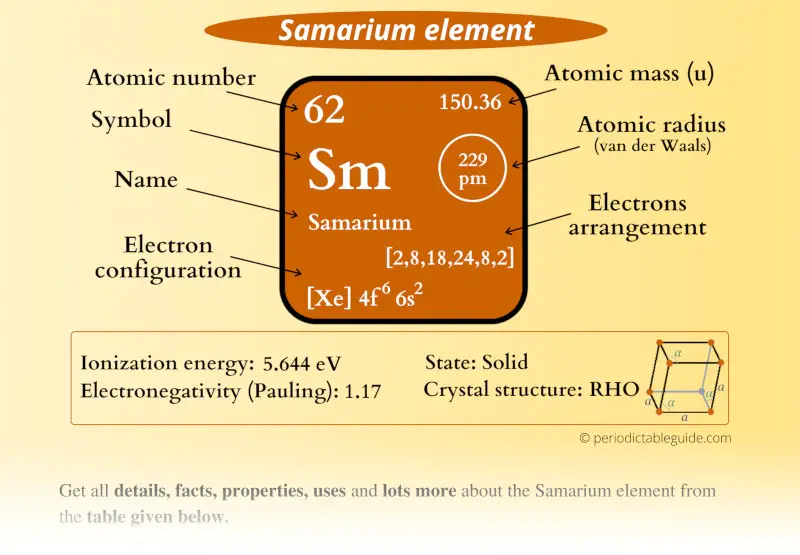
Samarium Element (Sm) Information
| Appearance |  Silvery white |
| State (at STP) | Solid |
| Position in Periodic table | 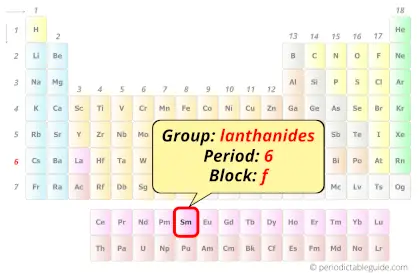 Group: lanthanides, Period: 6, Block: f |
| Category |  Inner transition metals |
| Atomic number or Protons | 62 |
| Neutrons | 88 |
| Electrons | 62 |
| Symbol | Sm |
| Atomic mass | 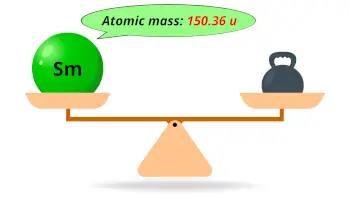 150.36 u |
| Electrons arrangement or Bohr model | 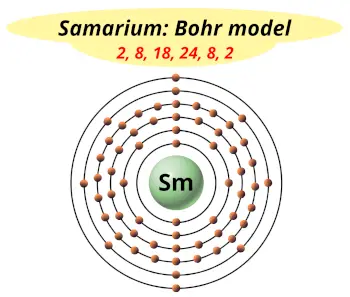 2, 8, 18, 24, 8, 2 |
| Electronic configuration | [Xe] 4f6 6s2 |
| Atomic radius | 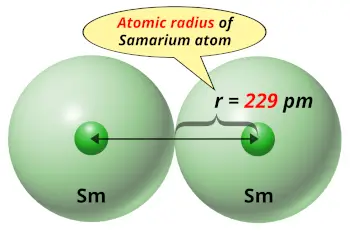 229 picometers (van der Waals radius) |
| 1st Ionization energy | 5.644 eV |
| Electronegativity | 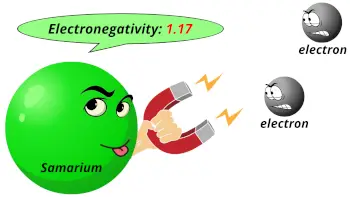 1.17 (Pauling scale) |
| Crystal structure | 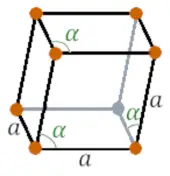 Rhombohedral |
| Melting point | 1345 K or 1072 °C or 1962 °F |
| Boiling point | 2173 K or 1900 °C or 3452 °F |
| Density | 7.35 g/cm3 |
| Main isotope | 152Sm |
| Who discovered Samarium and when? |  Lecoq de Boisbaudran in 1879 |
| CAS number | 7440-19-9 |
Samarium in Periodic table
Samarium element is in period 6 and in lanthanide group of the Periodic table. Samarium is the f-block element and it belongs to inner transition metals group.
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba | La* | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| *Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| **Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
←Move to: Promethium (Pm) element – Periodic Table
→Move to: Europium (Eu) element – Periodic Table
Why is Samarium in Period 6?
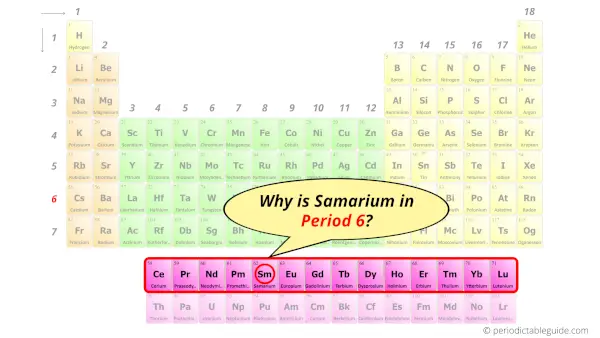
Let me ask you a question.
How many shells does samarium have?
It’s 6. Right?
You have already seen the bohr model of samarium atom in the above table.
From the Bohr model, it can be found that the number of orbits or shells in samarium is 6. Hence, as samarium has 6 orbits, it lies in period 6 of the Periodic table.
Why is Samarium in f-block?
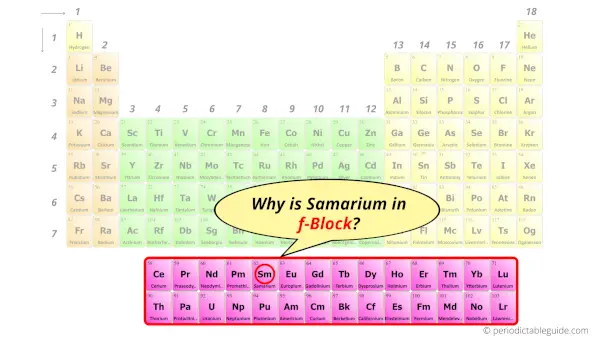
Before knowing this reason, first of all I want to ask you a simple question.
How can you determine the blocks-wise position of elements?
The simple answer: The elements will lie in the s, p, d or f block will completely depend upon the subshell in which the last electron will enter.
For example; the electron configuration of samarium is [Xe] 6s2 4f6.
So the last electron of samarium enters the f-subshell or f-orbital.
Hence, samarium is the f-block element.
5 Interesting facts about Samarium
Interesting facts about samarium element are mentioned below.
- The samarium element is obtained from its mineral named “samarskite”. Hence it was named “samarium”.
- This element was discovered by chemist Lecoq de Boisbaudran in 1879.
- Samarium element is found from the earth’s crust along with other rare earth elements.
- Samarium is the 40th most abundant element found on the earth.
- The abundance of samarium in earth’s crust is approximately 6 parts per million by weight.
Properties of Samarium
The physical and chemical properties of samarium element are mentioned below.
Physical properties of Samarium
Physical properties of samarium are mentioned below.
- Samarium is a solid metal having a silvery white appearance.
- The melting point and boiling point of samarium are 1072 °C and 1900 °C respectively.
- The atomic mass of samarium is 150.36 u and its density is 7.35 g/cm3.
- At room temperature, the crystal structure of samarium is rhombohedral.
- There are many isotopes of samarium, but out of these isotopes, the most abundant isotope is 152Sm (with an abundance of approximately 26.7%).
Chemical properties of Samarium
Chemical properties of samarium are mentioned below.
- Samarium is a reactive rare earth metal and it tarnishes in the air.
- As samarium is a chemically reactive metal, it is not found free in nature. But it is always found as a compound with other elements in the earth’s crust.
- When samarium is heated in the air at the temperature of 150 °C, it will ignite.
- If we see the electronic configuration of samarium, then the last electron in samarium enters the f-orbital and hence it is classified as a f-block element on the periodic table.
- The common oxidation state of samarium is +3, which is the same for most of the lanthanides.
- The first ionization energy of samarium is 5.644 eV.
Uses of Samarium
Uses of samarium are mentioned below.
- Samarium is used in carbon arc lighting that is used in studio lightings.
- Samarium is also used in preparing magnets. This is done by alloying samarium with cobalt.
- The magnets made from the alloy of cobalt and samarium are also used in devices like headphones, motors, etc.
- In nuclear power plants, samarium is used as a neutron absorber.
- Apart from these, it is also used in optical lasers as well as infrared absorbing glasses.
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
External resources:
- Samarium – Wikipedia. (2009, June 6). Samarium – Wikipedia. https://en.wikipedia.org/wiki/Samarium
- Samarium – Element information, properties and uses | Periodic Table. (n.d.). Samarium – Element Information, Properties and Uses | Periodic Table. https://www.rsc.org/periodic-table/element/62/samarium
- P. (n.d.). Samarium | Sm (Element) – PubChem. Samarium | Sm (Element) – PubChem. https://pubchem.ncbi.nlm.nih.gov/element/Samarium
- It’s Elemental – The Element Samarium. (n.d.). It’s Elemental – the Element Samarium. https://education.jlab.org/itselemental/ele062.html
