This is a SUPER easy guide on Plutonium element.
In fact, the table mentioned below is the perfect information box (Which gives you every single detail about the Plutonium element in Periodic table.)
So if you want to know anything about Plutonium element, then this guide is for you.
Let’s dive right into it!
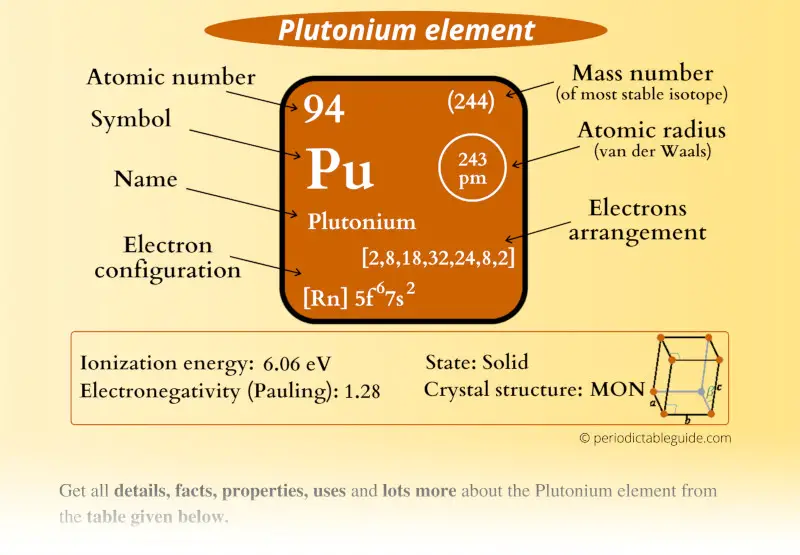
Plutonium Element (Pu) Information
| Appearance |  Silvery-white metallic |
| State (at STP) | Solid |
| Position in Periodic table | 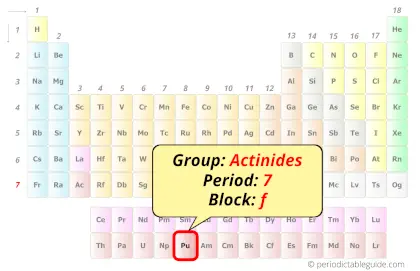 Group: actinides, Period: 7, Block: f |
| Category | 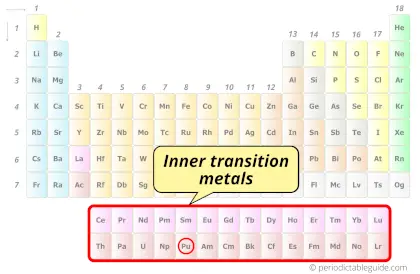 Inner transition metals |
| Atomic number or Protons | 94 |
| Neutrons | 150 |
| Electrons | 94 |
| Symbol | Pu |
| Atomic mass of Plutonium (most stable isotope) | 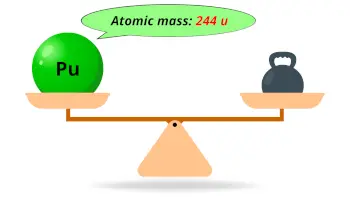 244 u |
| Electrons arrangement or Bohr model | 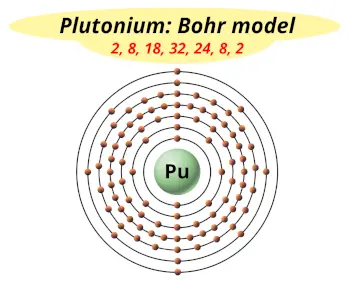 2, 8, 18, 32, 24, 8, 2 |
| Electronic configuration | [Rn] 5f6 7s2 |
| Atomic radius | 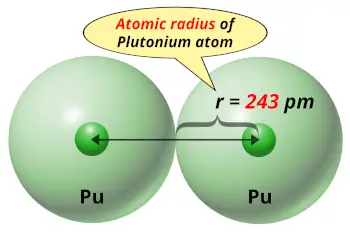 243 picometers (van der Waals radius) |
| 1st Ionization energy | 6.06 eV |
| Electronegativity | 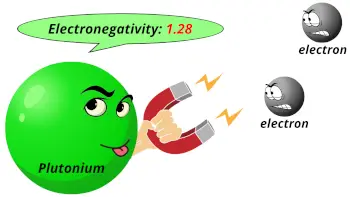 1.28 (Pauling scale) |
| Crystal structure | 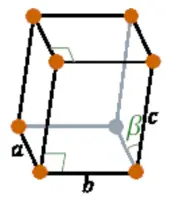 Monoclinic |
| Melting point | 912 K or 639 °C or 1183 °F |
| Boiling point | 3505 K or 3228 °C or 5842 °F |
| Density | 19.82 g/cm3 |
| Main isotopes | 238Pu, 239Pu and 240Pu |
| Who discovered Plutonium and when? | Glenn T. Seaborg, Edwin McMillan, Joseph W. Kennedy and Arthur Wahl (between 1940-41) |
| CAS number | 7440-07-5 |
Plutonium in Periodic table
Plutonium element is in period 7 and in actinides group of the Periodic table. Plutonium is the f-block element and it belongs to inner transition metals group.
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba | La* | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| *Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| **Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
←Move to: Neptunium (Np) element – Periodic Table
→Move to: Americium (Am) element – Periodic Table
Why is Plutonium in Period 7?
Let me ask you a question.
How many shells does plutonium have?
It’s 7. Right?
You have already seen the bohr model of plutonium atom in the above table.
From the Bohr model, it can be found that the number of orbits or shells in plutonium is 7. Hence, as plutonium has 7 orbits, it lies in period 7 of the Periodic table.
Why is Plutonium in f-block?
Before knowing this reason, first of all I want to ask you a simple question.
How can you determine the blocks-wise position of elements?
The simple answer: The elements will lie in the s, p, d or f block will completely depend upon the subshell in which the last electron will enter.
For example; the electron configuration of plutonium is [Rn] 5f6 7s2.
So the last electron of plutonium enters the f-subshell or f-orbital.
Hence, plutonium is the f-block element.
5 Interesting facts about Plutonium
Interesting facts about plutonium element are mentioned below.
- The name “Plutonium” was derived from the name of a dwarf planet “Pluto”.
- Plutonium was discovered by Glenn T. Seaborg, Edwin McMillan, Joseph W. Kennedy and Arthur Wahl (between 1940-41).
- Plutonium has around 20 isotopes, and all those isotopes are radioactive in nature.
- Plutonium is naturally available in very less quantities from the uranium ores. But for commercial purposes, it is artificially prepared from Uranium-238 in nuclear reactors.
- Out of all the isotopes of plutonium, the isotope 244Pu is the longest lived isotope which has a half life of 80.8 million years.
Properties of Plutonium
The physical and chemical properties of plutonium element are mentioned below.
Physical properties of Plutonium
Physical properties of plutonium are mentioned below.
- Plutonium is a solid metal having a silvery-white metallic appearance.
- Plutonium is a bad conductor of heat and electricity.
- The melting point and boiling point of plutonium are 639 °C and 3228 °C respectively.
- The crystal structure of plutonium is Monoclinic.
- The density of plutonium is more in liquid state as compared to that in solid state. [1]
Chemical properties of Plutonium
Chemical properties of plutonium are mentioned below.
- Plutonium is a radioactive element and it is harmful for all living beings.
- When plutonium is exposed to air, it reacts with oxygen and tarnishes easily. This forms a yellow oxide layer on it.
- Plutonium also forms compounds with elements like silicon, nitrogen, carbon, as well as halogens (like fluorine, chlorine, bromine, etc.)
- In compound form, Plutonium shows many oxidation states ranging from +2 to +8. Common oxidation states are +2, +3 and +4.
Uses of Plutonium
The uses of plutonium are mentioned below.
- The isotope 239Pu can undergo chain reaction, so it is used in nuclear weapons as well as nuclear reactors.
- The isotope 238Pu is used in radioisotope thermoelectric generators.
- Out of the total amount of energy produced in the nuclear power plants, around 1/3rd of this energy comes from plutonium.
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
External resources:
- Plutonium – Nuclear Museum. (2014, June 5). Nuclear Museum. https://ahf.nuclearmuseum.org/ahf/history/plutonium/
- P. (n.d.). Plutonium | Pu (Element) – PubChem. Plutonium | Pu (Element) – PubChem. https://pubchem.ncbi.nlm.nih.gov/element/Plutonium
- Backgrounder on Plutonium. (n.d.). NRC Web. https://www.nrc.gov/reading-rm/doc-collections/fact-sheets/plutonium.html
- It’s Elemental – The Element Plutonium. (n.d.). It’s Elemental – the Element Plutonium. https://education.jlab.org/itselemental/ele094.html
- S. (2022, October 11). Plutonium: Facts about the radioactive element. Space.com. https://www.space.com/what-is-plutonium
- Manhattan Project: Science > Nuclear Physics > PLUTONIUM CHEMISTRY AND METALLURGY. (n.d.). Manhattan Project: Science > Nuclear Physics > PLUTONIUM CHEMISTRY AND METALLURGY. https://www.osti.gov/opennet/manhattan-project-history/Science/NuclearPhysics/plutonium-chemistry.html
- Physical, Nuclear, and Chemical Properties of Plutonium – Institute for Energy and Environmental Research. (n.d.). Physical, Nuclear, and Chemical Properties of Plutonium – Institute for Energy and Environmental Research. https://ieer.org/resource/factsheets/plutonium-factsheet/
