This is a SUPER easy guide on Antimony element.
In fact, the table mentioned below is the perfect information box (Which gives you every single detail about the Antimony element in Periodic table.)
So if you want to know anything about Antimony element, then this guide is for you.
Let’s finish this very quickly.
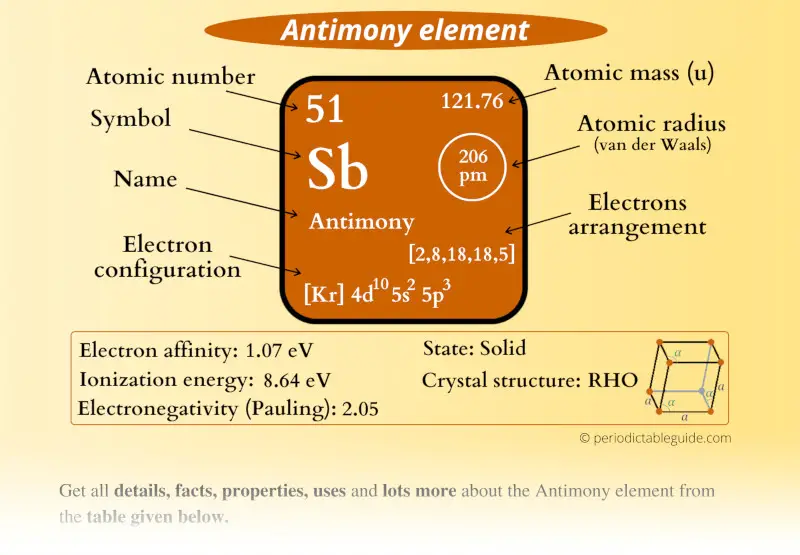
Antimony Element (Sb) Information
| Appearance |  Silvery gray metallic luster |
| State (at STP) | Solid |
| Position in Periodic table | 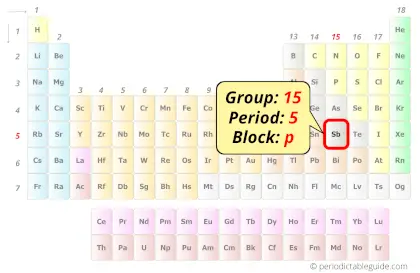 Group: 15, Period: 5, Block: p |
| Category |  Pnictogens |
| Atomic number or Protons | 51 |
| Neutrons | 71 |
| Electrons | 51 |
| Symbol | Sb |
| Atomic mass | 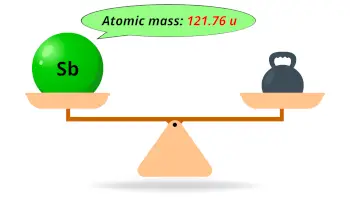 121.76 u |
| Electrons arrangement or Bohr model | 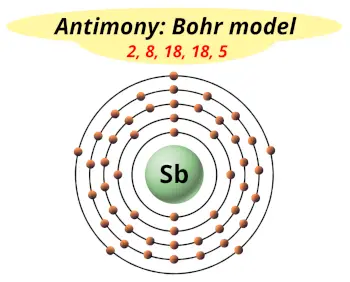 2, 8, 18, 18, 5 |
| Electronic configuration | [Kr] 4d10 5s2 5p3 |
| Atomic radius | 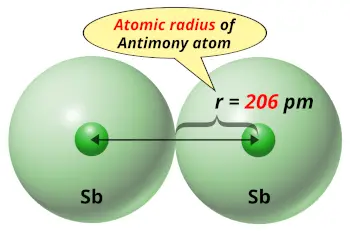 206 picometers (van der Waals radius) |
| Valence electrons | 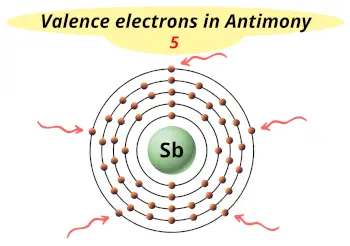 5 |
| 1st Ionization energy | 8.64 eV |
| Electronegativity | 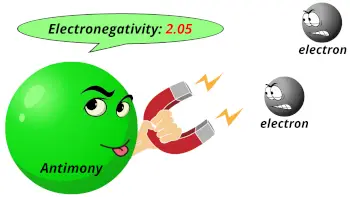 2.05 (Pauling scale) |
| Crystal structure | 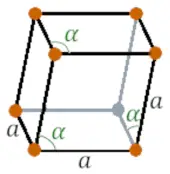 Rhombohedral |
| Melting point | 903.7 K or 630.6 °C or 1167.1 °F |
| Boiling point | 1908 K or 1635 °C or 2975 °F |
| Density | 6.7 g/cm3 |
| Main isotope | 121Sb |
| CAS number | 7440-36-0 |
Antimony in Periodic table
Antimony element is in group 15 and period 5 of the Periodic table. Antimony is the p-block element and it belongs to pnictogens group.
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba | La* | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| *Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| **Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
←Move to: Tin (Sn) element – Periodic Table
→Move to: Tellurium (Te) element – Periodic Table
Why is Antimony in Group 15?
Do you know, how many electrons can be accommodated in the first shell, second shell, third shell, fourth shell, etc…?
Here is the table showing the capacity of orbits to hold electrons.
Number of electrons in shells.
| Orbit / Shell (n) | Maximum no. of electrons this orbit can hold (2 × n2) |
| K shell, n = 1 | 2 × 1² = 2 |
| L shell, n = 2 | 2 × 2² = 8 |
| M shell, n = 3 | 2 × 3² = 18 |
| N shell, n = 4 | 2 × 4² = 32 |
| . . . | . . . |
Thus,
- 1st shell can hold 2 electrons.
- 2nd shell can hold 8 electrons.
- 3rd shell can hold 18 electrons.
- 4th shell can hold 32 electrons, and so on.
Now the atomic number of antimony (Sb) is 51.
Hence the electron arrangement in antimony is 2, 8, 18, 18, 5. And the electron configuration of antimony is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p3.
This electron arrangement and electron configuration indicates that the outermost orbit (i.e orbit number 5) of antimony atom has 5 electrons.
Hence, it lies in group 15.
Why is Antimony in Period 5?
Let me ask you a question.
How many shells does antimony have?
It’s 5. Right?
You have already seen the bohr model of antimony atom in the above table.
From the Bohr model, it can be found that the number of orbits or shells in antimony is 5. Hence, as antimony has 5 orbits, it lies in period 5 of the Periodic table.
Why is Antimony in p-block?
Before knowing this reason, first of all I want to ask you a simple question.
How can you determine the blocks-wise position of elements?
The simple answer: The elements will lie in the s, p, d or f block will completely depend upon the subshell in which the last electron will enter.
For example; the electron configuration of antimony is [Kr] 4d10 5s2 5p3.
So the last electron of antimony enters the p-subshell or p-orbital.
Hence, antimony is the p-block element.
5 Interesting facts about Antimony
Interesting facts about antimony element are mentioned below.
- The name antimony was derived from the Greek words “anti” and “monos” which means a metal that is not found alone.
- Antimony is very rarely found in its pure form, but it is mostly found from the mineral stibnite.
- The abundance of antimony in the earth’s crust is estimated to be 0.2 to 0.5 part per million.
- China is the leading producer and supplier of antimony in the world (more than 80%).
- In ancient times, antimony was used as a reflecting surface in mirrors.
Properties of Antimony
The physical and chemical properties of antimony element are mentioned below.
Physical properties of Antimony
Physical properties of antimony are mentioned below.
- Antimony is solid at STP and has a silvery grey metallic lustre.
- Antimony is a poor conductor of heat and electricity at room temperature.
- Antimony is hard and brittle which can not be malleable.
- The atomic mass of antimony is 121.76 u and its density is 6.7 g/cm3.
- The melting point of antimony is 630.6 °C and its boiling point is 1635 °C.
- The crystal structure of antimony is Rhombohedral.
- Antimony has many isotopes, but out of them the most abundant isotope is 121Sb (around 57%).
Chemical properties of Antimony
Chemical properties of antimony are mentioned below.
- When antimony is kept open in air, it does not react easily. The black allotrope of antimony easily gets corroded when kept open in air.
- When antimony is reacted with acids, it does not easily dissolve into it.
- Antimony can be dissolved in oxidizing acids like sulfuric acid or nitric acid.
- When antimony is heated in air, it forms antimony trioxide.
Uses of Antimony
Uses of antimony are mentioned below.
- Antimony is a metalloid and hence it is used in semiconductor devices like diodes, infrared detectors, etc.
- The compounds of antimony are used in manufacturing of glass, paints, ceramics, etc.
- The alloy formed by mixing antimony and lead is used in manufacturing battery components, cable sheathing, etc.
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
External resources:
- Antimony – Wikipedia. (2009, October 16). Antimony – Wikipedia. https://en.wikipedia.org/wiki/Antimony
- Antimony – Element information, properties and uses | Periodic Table. (n.d.). Antimony – Element Information, Properties and Uses | Periodic Table. https://www.rsc.org/periodic-table/element/51/antimony
- It’s Elemental – The Element Antimony. (n.d.). It’s Elemental – the Element Antimony. https://education.jlab.org/itselemental/ele051.html
- P. (n.d.). Antimony | Sb (Element) – PubChem. Antimony | Sb (Element) – PubChem. https://pubchem.ncbi.nlm.nih.gov/element/Antimony
- C&EN: IT’S ELEMENTAL: THE PERIODIC TABLE – ANTIMONY. (n.d.). C&EN: IT’S ELEMENTAL: THE PERIODIC TABLE – ANTIMONY. https://pubsapp.acs.org/cen/80th/antimony.html?
