This is a SUPER easy guide on Lithium element.
In fact, the table mentioned below is the perfect information box (Which gives you every single detail about the Lithium element in Periodic table.)
So if you want to know anything about the Lithium element, then this guide is for you.
Let’s finish this very quickly.
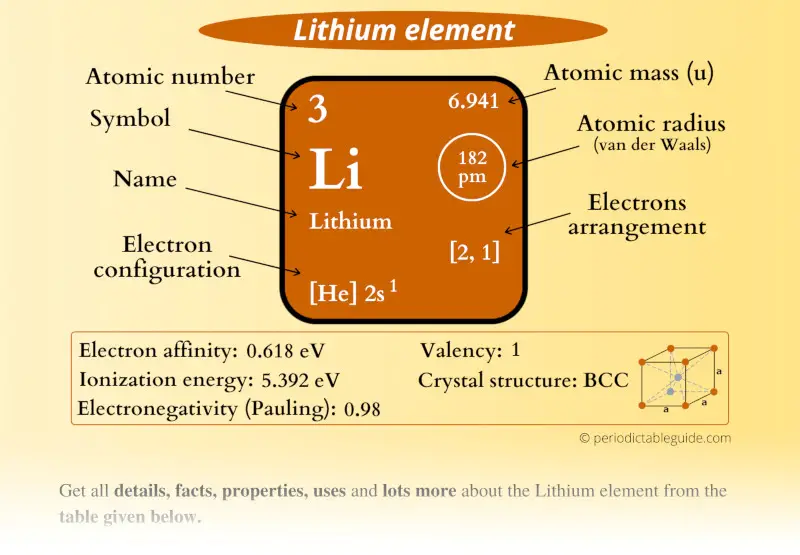
Lithium Element (Li) Information
| Appearance |  Silvery white |
| State (at STP) | Solid |
| Position in Periodic table |  Group: 1, Period: 2, Block: s |
| Category |  Alkali metals |
| Atomic number or Protons | 3 |
| Neutrons | 4 |
| Electrons | 3 |
| Symbol | Li |
| Atomic mass |  6.941 u |
| Electrons arrangement or Bohr model | 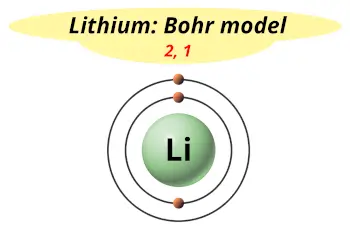 2, 1 |
| Electronic configuration | [He] 2s1 |
| Atomic radius |  182 picometers (van der Waals radius) |
| Valence electrons |  1 |
| 1st Ionization energy | 5.392 eV |
| Electronegativity |  0.98 (Pauling scale) |
| Crystal structure |  BCC (Body Centered Cubic) |
| Melting point | 453.65 K or 180.5 °C or 356.9 °F |
| Boiling point | 1603 K or 1330 °C or 2426 °F |
| Density | 0.534 g/cm3 |
| Main isotope | 7Li |
| Who discovered Lithium and when? |  John August Arfwedson in 1817 |
| CAS number | 7439-93-2 |
Lithium in Periodic table
Lithium element is in group 1 and period 2 of the Periodic table. Lithium is the s-block element and it belongs to alkali metals group.
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba | La* | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| *Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| **Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
←Move to: Helium (He) element – Periodic Table
→Move to: Beryllium (Be) element – Periodic Table
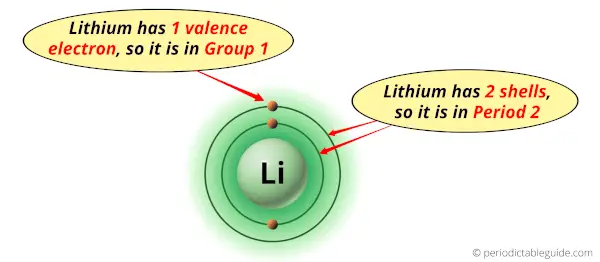
Why is Lithium in Group 1 and Period 2 of the Periodic table?
Lithium is in group 1 because it has 1 valence electron.
Lithium is in period 2 because it has 2 shells or orbits.
But have you ever wondered, why is Lithium in group 1? Why is it in period 2? And why is it in s-block?
I’ll explain to you the perfect detailed reason behind this.
Let’s see the reasons one by one.
Why is Lithium in Group 1?

Do you know, how many electrons can be accommodated in the first shell, second shell, third shell, fourth shell, etc…?
Here is the table showing the capacity of orbits to hold electrons.
Number of electrons in shells.
| Orbit / Shell (n) | Maximum no. of electrons this orbit can hold |
| K shell, n = 1 | 2 × 1² = 2 |
| L shell, n = 2 | 2 × 2² = 8 |
| M shell, n = 3 | 2 × 3² = 18 |
| N shell, n = 4 | 2 × 4² = 32 |
Thus,
- 1st shell can hold 2 electrons.
- 2nd shell can hold 8 electrons.
- 3rd shell can hold 18 electrons.
- 4th shell can hold 32 electrons.
The atomic number of Lithium (Li) is 3. Hence, Lithium element has the electrons arrangement 2, 1. This electron arrangement indicates that the outermost orbit of Lithium (Li) has 1 electron. Hence, Lithium lies in group 1.
Why is Lithium in Period 2?
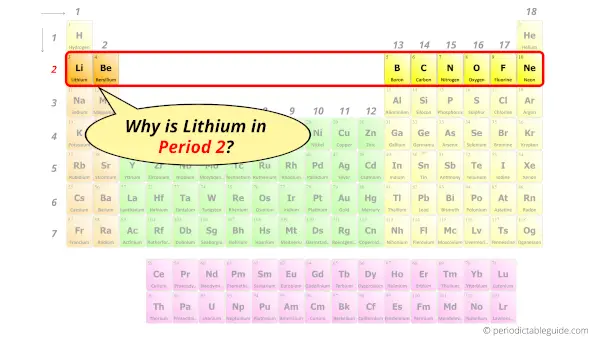
Let me ask you a question.
How many shells does Lithium have?
It’s 2. Right?
You have already seen the bohr model of Lithium element in the above table and you have seen that the number of orbits or shells in Lithium is 2.
Hence, as it has 2 orbits, it lies in the 2nd period of the Periodic table.
Why is Lithium in s-block?
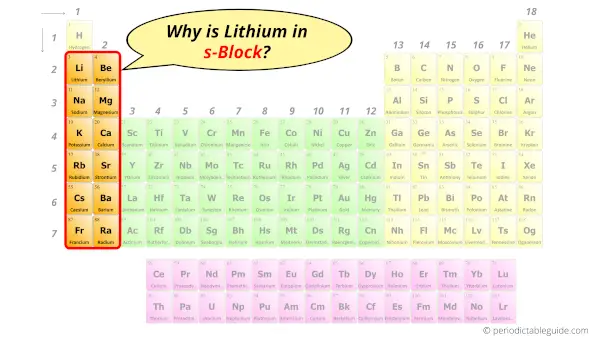
Before knowing this reason, first of all a simple question to you.
How can you determine the blocks wise position of elements?
The simple answer: The elements will lie in the s, p, d or f block will completely depend upon the subshell in which the last electron will enter.
For example; the electron configuration of Lithium is [He] 2s1.
So the last electron of lithium enters the s-subshell or s-orbital.
Hence, Lithium is the s-block element.
Is Lithium a Metal or Nonmetal?
Yes, Lithium is a metal having silvery-white color. Lithium is the lightest metal and it even floats on water. It is soft metal which can be cut even with a butter knife.

But I want to ask you a simple question.
Do you know what exactly the metals are?
Answer:
They are the substance which gives or donates electrons during a chemical reaction.
Lithium has one electron in its outermost orbit.
During the chemical reaction, it donates or gives this valence electron to the element with which it combines.

Hence, this indicates that lithium is a metal.
Also see: Location of all the metals on the Periodic table (Image)
Why Lithium floats on water?

Simple reason: Lithium is lighter than water, hence it floats on water.
Explanation:
Let me give you a proper explanation why Lithium floats on water.
You might know about the property called “density”.
Density is a major factor which decides whether the object will float or sink.
If the substance is more dense than water, then it will sink in water. And if the substance is less dense than water, then it will float on water.
Now, the density of Lithium is 0.534 g/cm3 and density of water is 1 g/cm3.
Thus, Lithium is lighter than water. Hence it floats on water.
Is Lithium reactive to water?
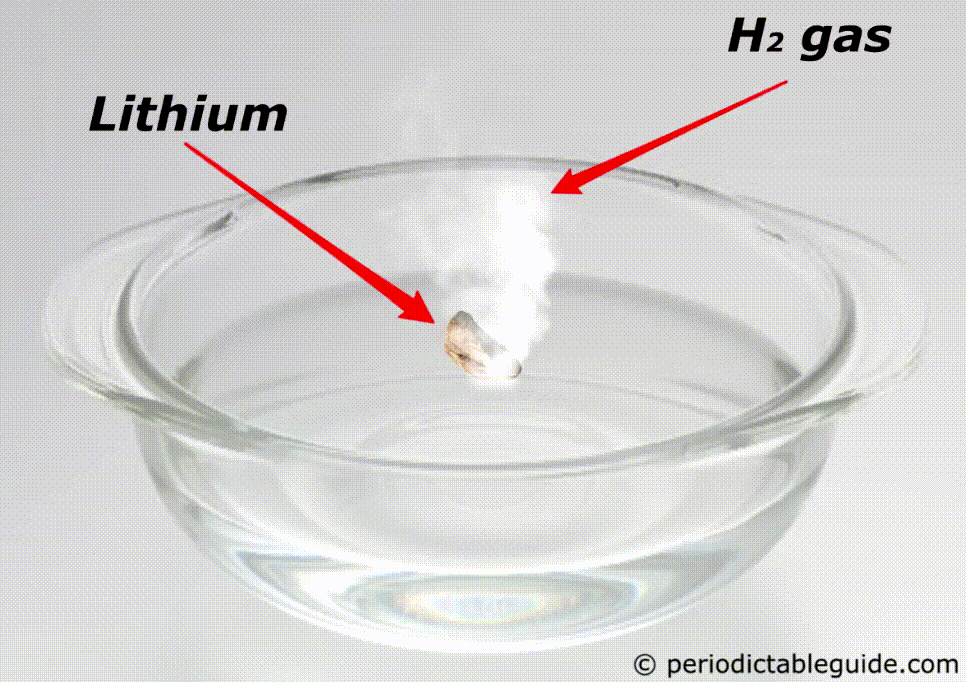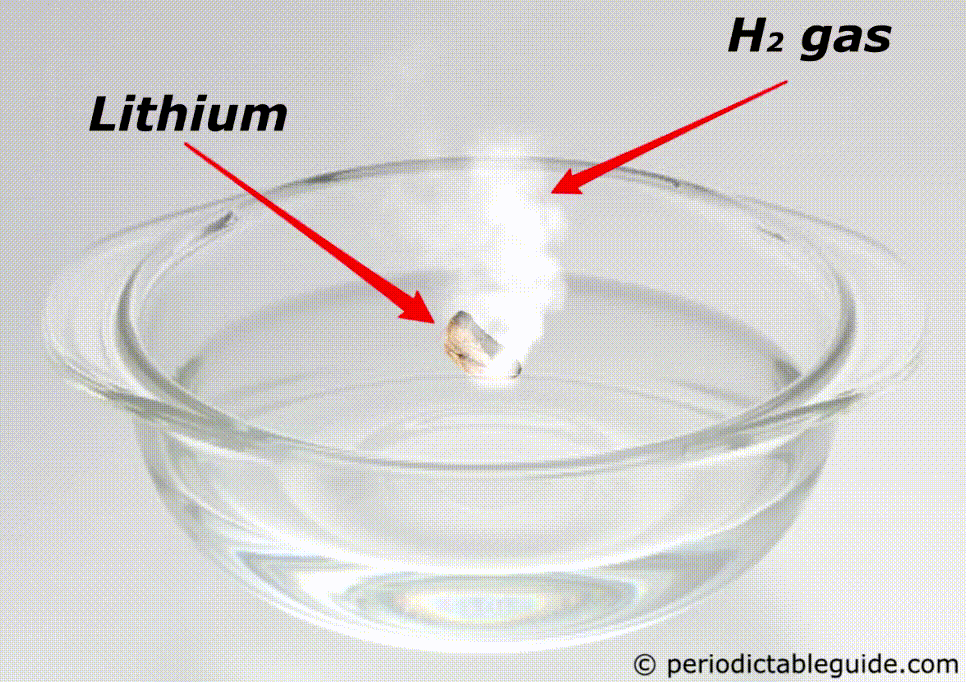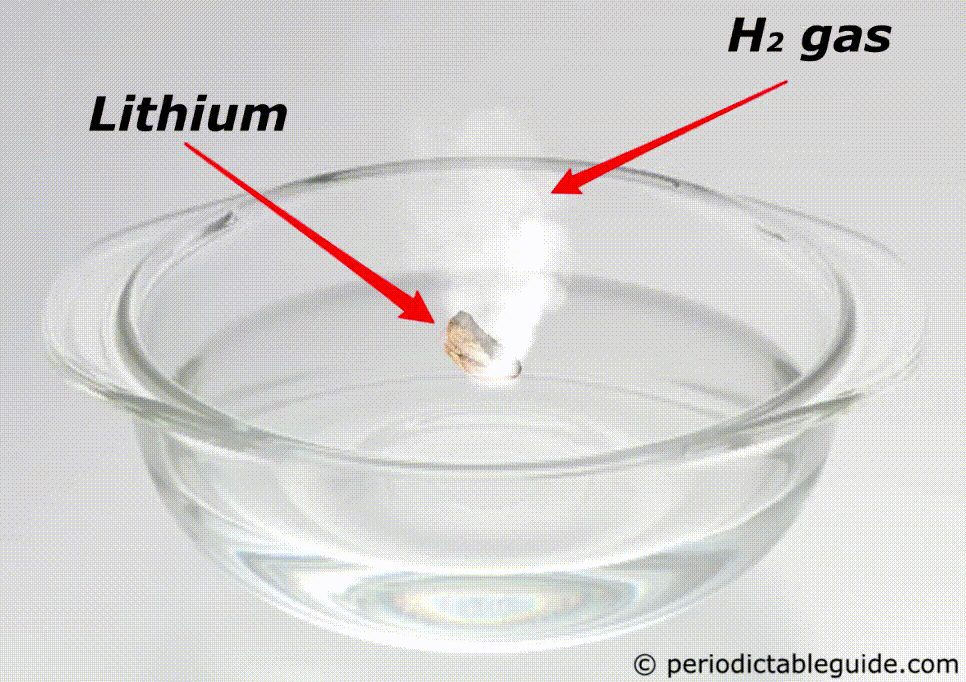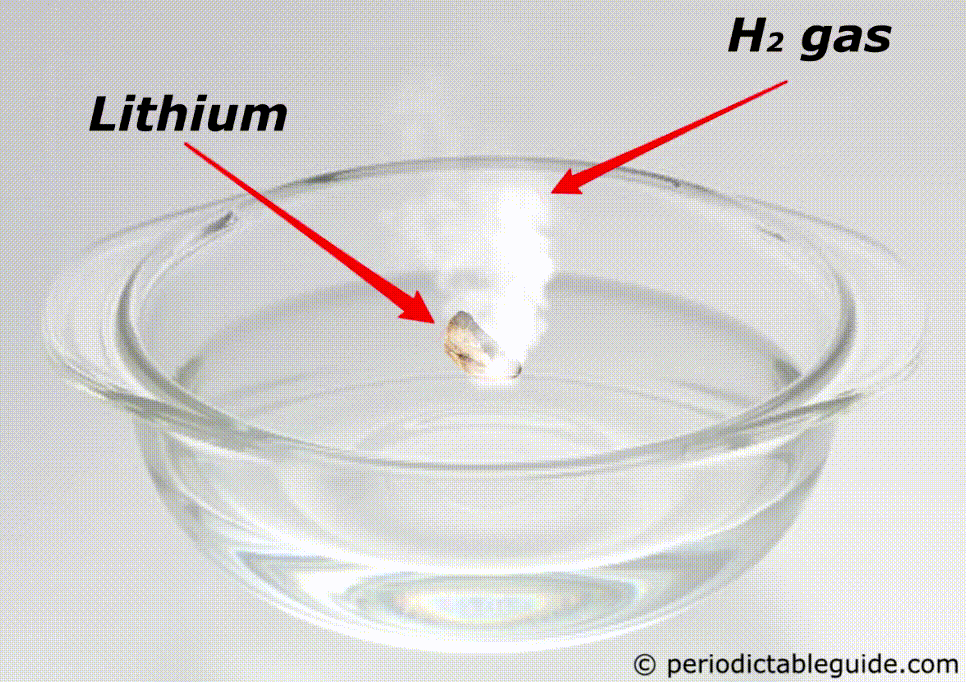
Yes, lithium is very reactive to water.
If lithium is kept in water, then the chemical reaction of lithium and water starts immediately which makes the solution Alkaline and liberates the hydrogen gas.
Here is the complete chemical equation for this reaction.
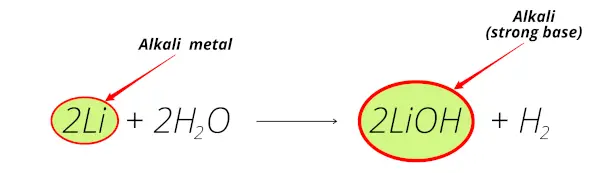
Even Lithium is so reactive that it does not exist as a pure metal in nature.
But it is always found in a compound form with other elements.
But the question is;
Why is Lithium so reactive?
Here is the reason.
The lithium atoms have only one valence electron, which is very easy to lose.
Plus, lithium is on the leftmost group of the Periodic table (group 1), and according to the Periodic trends, the atomic size decreases from left to right in a Periodic table.
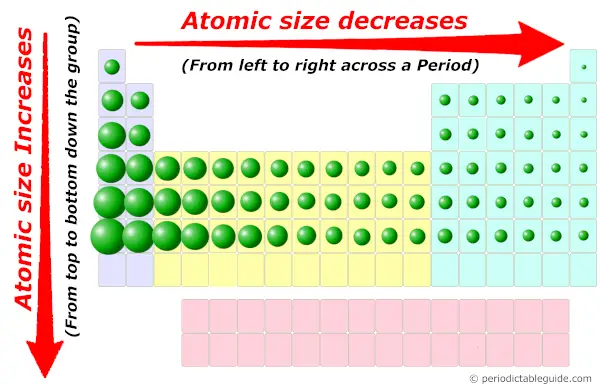
In other words, the elements which are on the left side of Periodic table have a bigger atomic size.
Hence, the size of Lithium atom is larger than other elements of the same period.
Due to large size, the attractive force between the nucleus and valence electrons will be less.

Hence, the loss of electrons becomes very easy.
This makes the lithium metal more reactive.
Also the Lithium element has very weak metallic bonds, which indicates that there is very less intermolecular attractive force.
Because of the weak intermolecular attractive force, the lithium atom loses the valence electrons very easily.
Hence from the above reasons, it can be said that lithium metal is more reactive.
Why does Lithium emit crimson red light on heating?

When Lithium metal is heated in a flame, its outermost electron gets excited onto a higher energy level.
When this excited electron loses its energy, it again comes back to the lower energy level.
During this phenomenon, it emits an electromagnetic radiation having a wavelength of around 670.8 nm.
This emitted radiation of 670.8 nm is nothing but a visible crimson red light.
Hence, lithium gives crimson red flame when it is heated.
Interesting Facts about Lithium
The 7 interesting facts about Lithium are mentioned below.
- Lithium is a metal, but it floats on water. Because it is lighter than water.
- If Lithium catches fire, then you cannot extinguish that fire using water. Because Lithium is reactive to water.
- Lithium produces crimson red flame when heated in a Bunsen burner.
- Lithium is a metal, but it is so soft that it can even be cut using a kitchen knife.
- Lithium is highly corrosive, so it is stored in kerosene oil or it is stored in an inert atmosphere.
- Density of Lithium is around half of that of waters’ density (Density of Lithium = ½ density of water).
- Lithium is so reactive that it is never found in its pure form, but it always exists along with other elements in a compound form.
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
External resources:
- Lithium – Element information, properties and uses | Periodic Table. (n.d.). Lithium – Element Information, Properties and Uses | Periodic Table. https://www.rsc.org/periodic-table/element/3/lithium
- P. (n.d.). Lithium | Li (Element) – PubChem. Lithium | Li (Element) – PubChem. https://pubchem.ncbi.nlm.nih.gov/element/Lithium
- It’s Elemental – The Element Lithium. (n.d.). It’s Elemental – the Element Lithium. https://education.jlab.org/itselemental/ele003.html
- C&EN: IT’S ELEMENTAL: THE PERIODIC TABLE – LITHIUM. (n.d.). C&EN: IT’S ELEMENTAL: THE PERIODIC TABLE – LITHIUM. https://pubsapp.acs.org/cen/80th/lithium.html?
