This is a SUPER easy guide on Molybdenum element.
In fact, the table mentioned below is the perfect information box (Which gives you every single detail about the Molybdenum element in Periodic table.)
So if you want to know anything about Molybdenum element, then this guide is for you.
Let’s finish this very quickly.
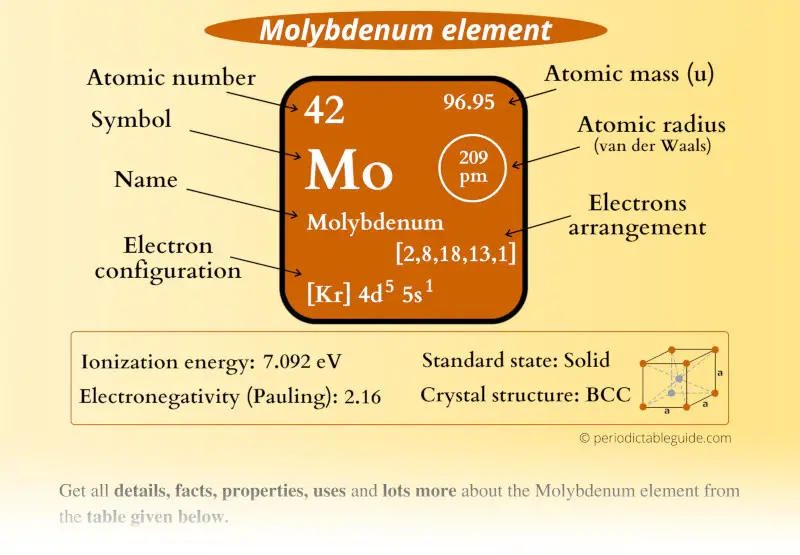
Molybdenum Element (Mo) Information
| Appearance |  Grey metallic luster |
| State (at STP) | Solid |
| Position in Periodic table | 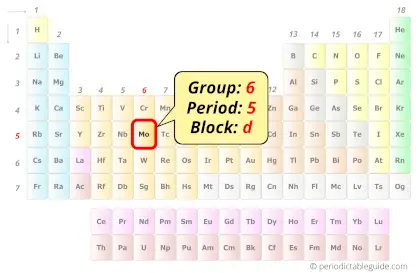 Group: 6, Period: 5, Block: d |
| Category | 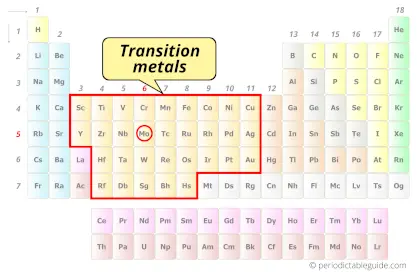 Transition metals |
| Atomic number or Protons | 42 |
| Neutrons | 54 |
| Electrons | 42 |
| Symbol | Mo |
| Atomic mass |  96.95 u |
| Electrons arrangement or Bohr model | 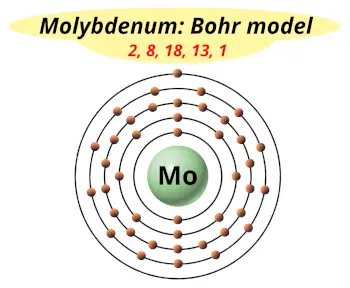 2, 8, 18, 13, 1 |
| Electronic configuration | [Kr] 4d5 5s1 |
| Atomic radius | 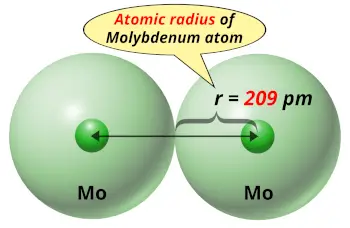 209 picometers (van der Waals radius) |
| 1st Ionization energy | 7.092 eV |
| Electronegativity | 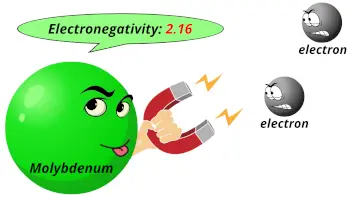 2.16 (Pauling scale) |
| Crystal structure | 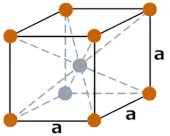 BCC (Body centered cubic) |
| Melting point | 2896 K or 2623 °C or 4753 °F |
| Boiling point | 4912 K or 4639 °C or 8382 °F |
| Density | 10.28 g/cm3 |
| Main isotopes | 95Mo, 96Mo, 98Mo |
| Who discovered Molybdenum and when? |  Carl Wilhelm Scheele in 1778 |
| CAS number | 7439-98-7 |
Molybdenum in Periodic table
Molybdenum element is in group 6 and period 5 of the Periodic table. Molybdenum is the d-block element and it belongs to Transition metals group.
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba | La* | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| *Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| **Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
←Move to: Niobium (Nb) element – Periodic Table
→Move to: Technetium (Tc) element – Periodic Table
Why is Molybdenum in Period 5?
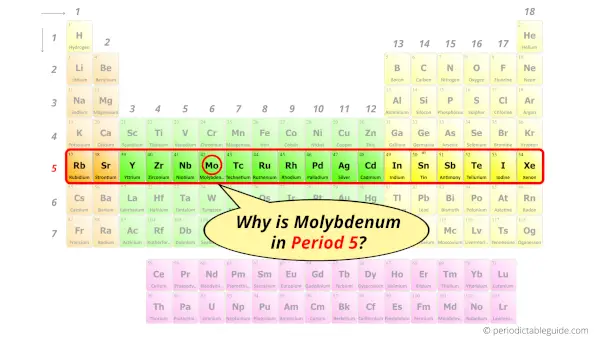
Let me ask you a question.
How many shells does molybdenum have?
It’s 5. Right?
You have already seen the bohr model of molybdenum atom in the above table.
From the Bohr model, it can be found that the number of orbits or shells in molybdenum is 5. Hence, as molybdenum has 5 orbits, it lies in period 5 of the Periodic table.
Why is Molybdenum in d-block?
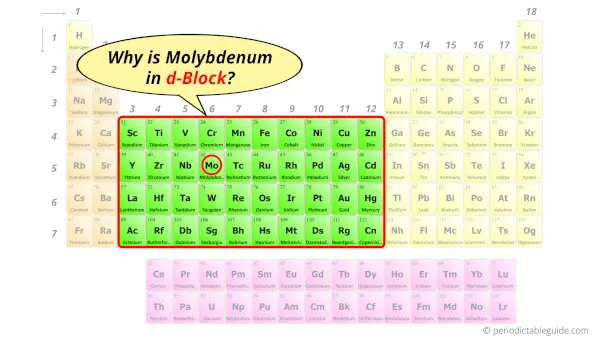
Before knowing this reason, first of all I want to ask you a simple question.
How can you determine the blocks-wise position of elements?
The simple answer: The elements will lie in the s, p, d or f block will completely depend upon the subshell in which the last electron will enter.
For example; the electron configuration of molybdenum is [Kr] 5s1 4d5.
So the last electron of molybdenum enters the d-subshell or d-orbital.
Hence, molybdenum is the d-block element.
Is Molybdenum a Transition Metal? Why?
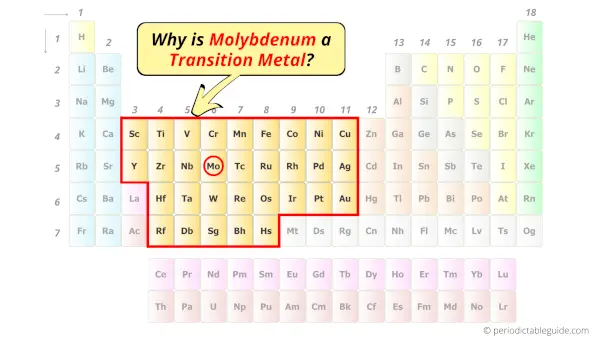
Yes, Molybdenum is a transition metal because it has incompletely filled d-orbital in its ground state.
Let me explain the exact meaning of this.
According to the definition of transition metals;
The element should compulsorily have incomplete d-orbitals, either in their ground state (M) or most common oxidation states (M1+, M2+, etc) then only they are called transition metals.
Now, the ground state of molybdenum means its normal state in which it has neither gained nor lost any electron/s.
So the ground state of molybdenum is Mo.
And the ground state electronic configuration of molybdenum is [Kr] 5s1 4d5.
In this state, if we see the electron configuration of molybdenum, then it possesses incomplete d-orbitals.
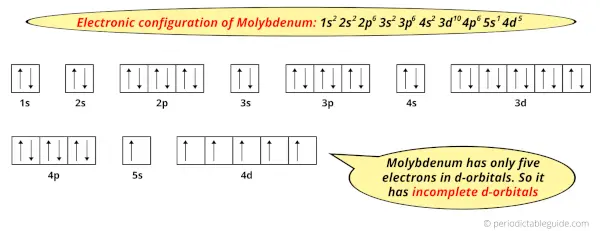
Because, there are only five electrons in the d-orbitals.
In order to have the complete d-orbitals, there must be 10 electrons in it.
But in the ground state electronic configuration of molybdenum, you can see that it has only 5 electrons in d-orbitals.
Thus, molybdenum has incomplete d-orbitals.
And hence, as molybdenum has incomplete d-orbitals, it is considered as a transition metal.
9 Interesting facts about Molybdenum
Interesting facts about molybdenum element are mentioned below.
- The name molybdenum was derived from the Greek word “molybdos” (which means lead).
- The proportion of molybdenum in the earth’s crust is 1.2 ppm by weight.
- Molybdenum is the 54th most common element in the earth’s crust.
- China is the leading producer of molybdenum ore in the world.
- Molybdenum is the micronutrient present in plants as well as animals.
- The annual production of molybdenum in the world is around 200,000 tons. [1]
- Before the discovery of molybdenum, its mineral molybdenite was mistaken as graphite or lead ore.
- Density of molybdenum is approximately half of that of tungsten.
- Almost 80% of the molybdenum is used in production of steels.
Properties of Molybdenum
The physical and chemical properties of molybdenum element are mentioned below.
Physical properties of Molybdenum
Physical properties of molybdenum are mentioned below.
- Molybdenum is a solid metal having Grey metallic lustre.
- The melting point of molybdenum is 2623 °C and its boiling point is 4639 °C.
- The atomic mass of molybdenum is 96.95 u and its density is 10.28 g/cm3.
- Molybdenum has many isotopes, but out of them the common isotopes are 95Mo (15.8 %), 96Mo (16.6 %), and 98Mo (24.3 %).
Chemical properties of Molybdenum
Chemical properties of molybdenum are mentioned below.
- Molybdenum has incomplete d-orbitals and hence it is classified as a transition metal on the periodic table.
- Molybdenum has many oxidation states and hence it can easily form compounds with other elements.
- Molybdenum does not react with water at room temperature, but it reacts with it at higher temperatures (above 600 °C) and forms molybdenum trioxide.
Uses of Molybdenum
Uses of molybdenum are mentioned below.
- Molybdenum is used as an alloying element with other metals to increase its strength, hardness and other properties.
- Molybdenum is also used in manufacturing of engine parts, missiles, drills , saw blades, etc.
- Molybdenum is generally available in a powdered form and it is also used in powder metallurgy.
- Molybdenum is also used in glass melting electrodes as it can resist high temperature.
- Molybdenum is also used as a catalyst in petroleum industries.
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
External resources:
- Molybdenum – Wikipedia. (2007, December 10). Molybdenum – Wikipedia. https://en.wikipedia.org/wiki/Molybdenum
- Molybdenum – Element information, properties and uses | Periodic Table. (n.d.). Molybdenum – Element Information, Properties and Uses | Periodic Table. https://www.rsc.org/periodic-table/element/42/molybdenum
- P. (n.d.). Molybdenum | Mo (Element) – PubChem. Molybdenum | Mo (Element) – PubChem. https://pubchem.ncbi.nlm.nih.gov/element/Molybdenum
- Molybdenum | CCDC. (n.d.). Molybdenum | CCDC. https://www.ccdc.cam.ac.uk/elements/molybdenum/
- Molybdenum – Energy Education. (n.d.). Molybdenum – Energy Education. https://energyeducation.ca/encyclopedia/Molybdenum
