This is a SUPER easy guide on Cobalt element.
In fact, the table mentioned below is the perfect information box (Which gives you every single detail about the Cobalt element in Periodic table.)
So if you want to know anything about Cobalt element, then this guide is for you.
Let’s dive right into it!
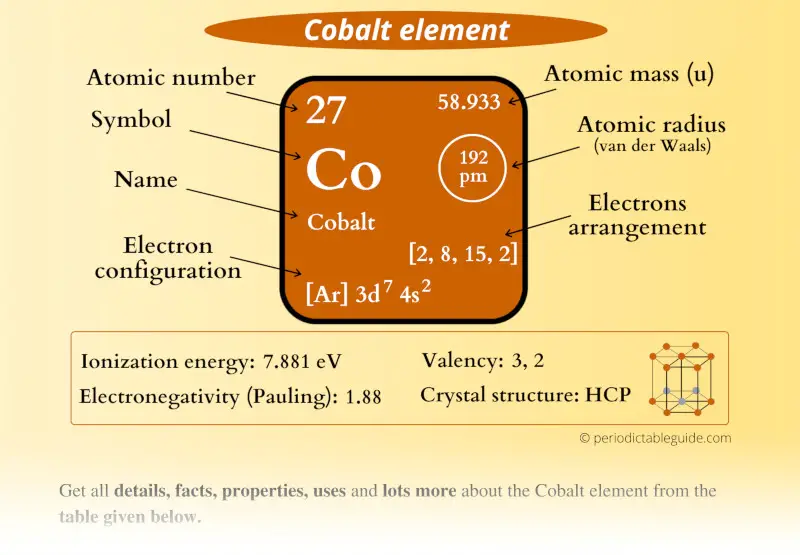
Cobalt Element (Co) Information
| Appearance |  Bluish lustrous grey metallic |
| State (at STP) | Solid |
| Position in Periodic table | 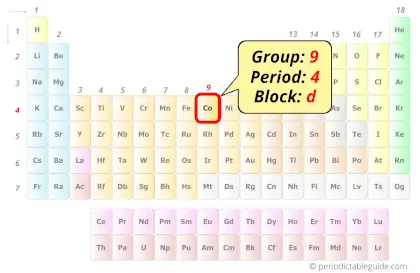 Group: 9, Period: 4, Block: d |
| Category | 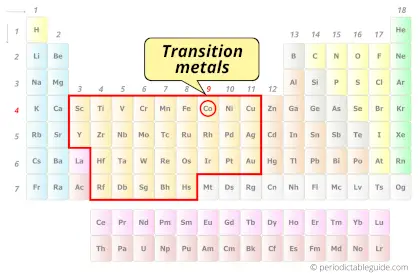 Transition metals |
| Atomic number or Protons | 27 |
| Neutrons | 32 |
| Electrons | 27 |
| Symbol | Co |
| Atomic mass |  58.933 u |
| Electrons arrangement or Bohr model | 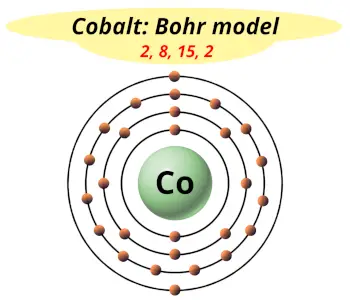 2, 8, 15, 2 |
| Electronic configuration | [Ar] 3d7 4s2 |
| Atomic radius | 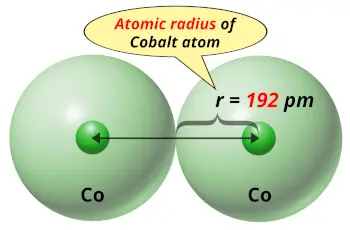 192 picometers (van der Waals radius) |
| 1st Ionization energy | 7.881 eV |
| Electronegativity | 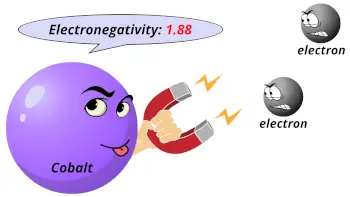 1.88 (Pauling scale) |
| Crystal structure | 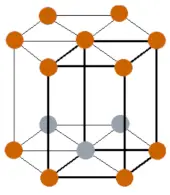 HCP (Hexagonal close packing) |
| Melting point | 1768 K or 1495 °C or 2723 °F |
| Boiling point | 3200 K or 2927 °C or 5301 °F |
| Density | 8.9 g/cm3 |
| Main isotope | 59Co |
| Who discovered Cobalt and when? | Georg Brandt in 1735 |
| CAS number | 7440-48-4 |
Cobalt in Periodic table
Cobalt element is in group 9 and period 4 of the Periodic table. Cobalt is the d-block element and it belongs to transition metals group.
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba | La* | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| *Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| **Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
←Move to: Iron (Fe) element – Periodic Table
→Move to: Nickel (Ni) element – Periodic Table
Why is Cobalt in Period 4?
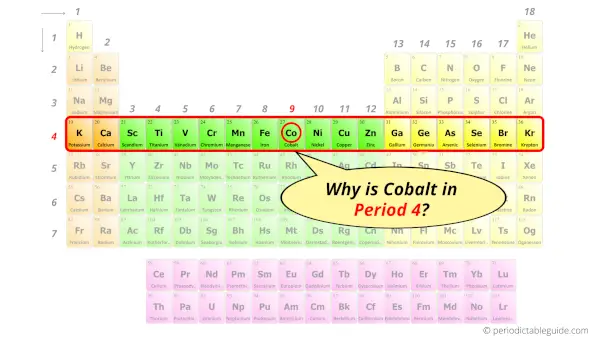
Let me ask you a question.
How many shells does cobalt have?
It’s 4. Right?
You have already seen the bohr model of cobalt atom in the above table.
From the Bohr model, it can be found that the number of orbits or shells in cobalt is 4. Hence, as cobalt has 4 orbits, it lies in period 4 of the Periodic table.
Why is Cobalt in d-block?
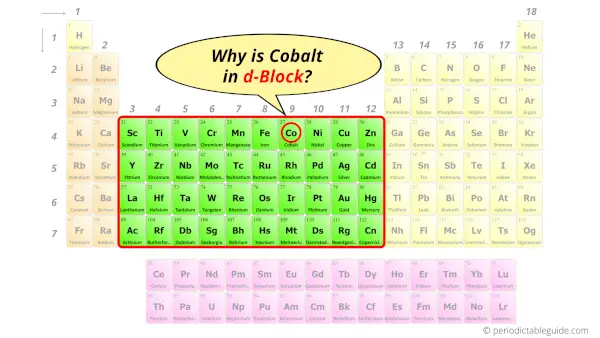
Before knowing this reason, first of all I want to ask you a simple question.
How can you determine the blocks-wise position of elements?
The simple answer: The elements will lie in the s, p, d or f block will completely depend upon the subshell in which the last electron will enter.
For example; the electron configuration of cobalt is [Ar] 4s2 3d7.
So the last electron of cobalt enters the d-subshell or d-orbital.
Hence, cobalt is the d-block element.
Is Cobalt a Transition Metal? Why?
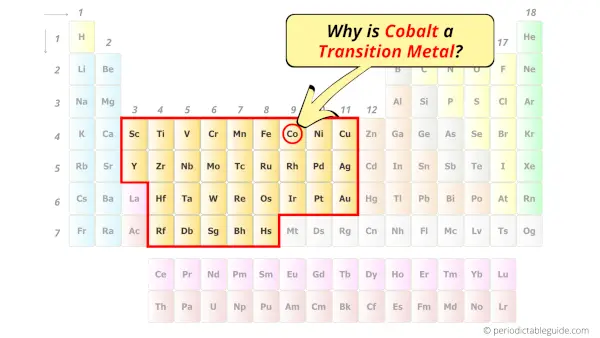
Yes, Cobalt is a transition metal because it has incompletely filled d-orbital in its ground state.
Let me explain the exact meaning of this.
According to the definition of transition metals;
The element should compulsorily have incomplete d-orbitals, either in their ground state (M) or most common oxidation states (M1+, M2+, etc) then only they are called transition metals.
Now, the ground state of Cobalt means its normal state in which it has neither gained nor lost any electron/s.
So the ground state of Cobalt is Co.
And the ground state electronic configuration of Cobalt is [Ar] 4s2 3d7.
In this state, if we see the electron configuration of Cobalt, then it possesses incomplete d-orbitals.
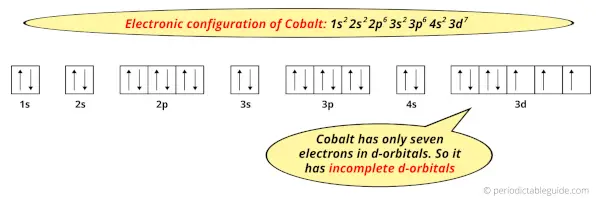
Because, there are only seven electrons in the d-orbitals.
In order to have the complete d-orbitals, there must be 10 electrons in it.
But in the ground state electronic configuration of cobalt, you can see that it has only 7 electrons in d-orbitals.
Thus, Cobalt has incomplete d-orbitals.
And hence, as Cobalt has incomplete d-orbitals, it is considered as a transition metal.
7 Interesting facts about Cobalt
Interesting facts about cobalt element are mentioned below.
- Cobalt is the name derived from the German word “kobald” (meaning “goblin”).
- The abundance of cobalt in earth’s crust is around 25 ppm (parts per million) by weight.
- Cobalt is one of the few metals that are naturally magnetic. Also cobalt can maintain the magnetism at high temperatures.
- Africa is the leading producer of cobalt in the world.
- Cobalt is also present in the human body, but its excess amount can be dangerous.
- Around 30% of cobalt produced nowadays is used in ceramics industries as well as paints industries.
- Cobalt is obtained as a byproduct while mining other metals (especially copper and nickel).
Properties of Cobalt
The physical and chemical properties of cobalt element are mentioned below.
Physical properties of Cobalt
Physical properties of cobalt are mentioned below.
- Cobalt is a transition metal having bluish lustrous grey metallic surface.
- The melting point of cobalt is 1495 °C and its boiling point is 2927 °C.
- There are various synthetic isotopes of cobalt, but the most abundant naturally occurring stable isotope is the 59Co.
- The atomic mass of cobalt is 58.933 u and its density is 8.9 g/cm3.
Chemical properties of Cobalt
Chemical properties of cobalt are mentioned below.
- As cobalt is fairly reactive, it is not found in a free state in nature. But it is always found as a compound with other elements.
- If cobalt is kept open in the air, it slowly reacts with the oxygen of the air and forms cobalt oxide.
- Cobalt has incomplete d-orbitals. Hence it is classified as a transition metal on the periodic table.
Uses of Cobalt
Uses of cobalt are mentioned below.
- Cobalt is used mainly as an alloying element in manufacturing of superalloys which possess anticorrosive properties as well as they are also stable at higher temperature.
- Cobalt is used in manufacturing inks, glass, paints that require a blue colouring agent.
- When cobalt combines with other elements, it can give other different colors. So it is used as a coloring agent in manufacturing various other materials.
- Cobalt has a property to maintain its magnetism even at higher temperatures. Hence cobalt is used to make magnets for generators, drives, and other devices that are subjected to higher temperature.
- Cobalt is also used in making rechargeable batteries.
- The alloys used for manufacturing aircraft engine parts need to be strong and heat resistant. So for such purposes, cobalt is used as an alloying metal for preparing such alloys.
- Radioactive isotope of cobalt (60Co) is used in medical applications to treat cancer tumors.
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
External resources:
- Cobalt. (n.d.). Cobalt. https://webbook.nist.gov/cgi/inchi?ID=C7440484&Mask=20
- C&EN: IT’S ELEMENTAL: THE PERIODIC TABLE – COBALT. (n.d.). C&EN: IT’S ELEMENTAL: THE PERIODIC TABLE – COBALT. https://pubsapp.acs.org/cen/80th/print/cobalt.html?
- It’s Elemental – The Element Cobalt. (n.d.). It’s Elemental – the Element Cobalt. https://education.jlab.org/itselemental/ele027.html
- P. (n.d.). Cobalt | Co (Element) – PubChem. Cobalt | Co (Element) – PubChem. https://pubchem.ncbi.nlm.nih.gov/element/Cobalt
- Cobalt – Wikipedia. (2008, September 19). Cobalt – Wikipedia. https://en.wikipedia.org/wiki/Cobalt
- Cobalt – Element information, properties and uses | Periodic Table. (n.d.). Cobalt – Element Information, Properties and Uses | Periodic Table. https://www.rsc.org/periodic-table/element/27/cobalt
