This is a SUPER easy guide on Beryllium element.
In fact, the table mentioned below is the perfect information box (Which gives you every single detail about the Beryllium element in Periodic table.)
So if you want to know anything about Beryllium element, then this guide is for you.
Let’s finish this very quickly.
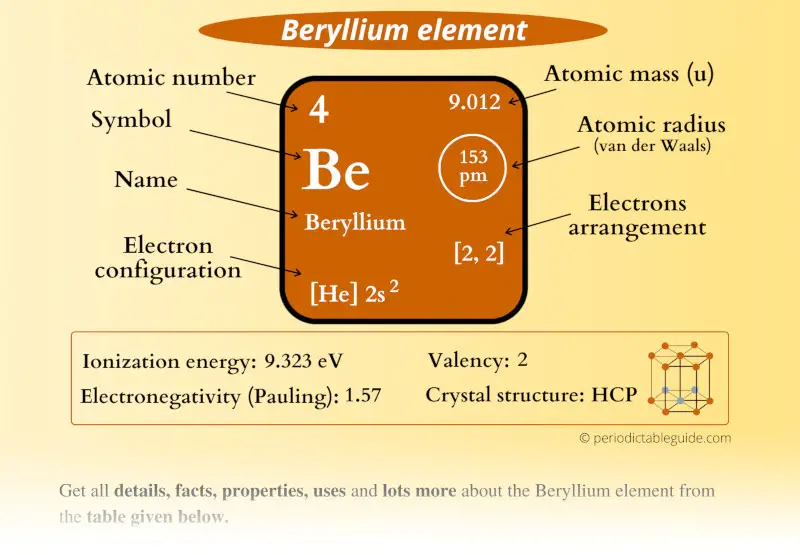
Beryllium Element (Be) Information
| Appearance |  Metallic white-grey |
| State (at STP) | Solid |
| Position in Periodic table | 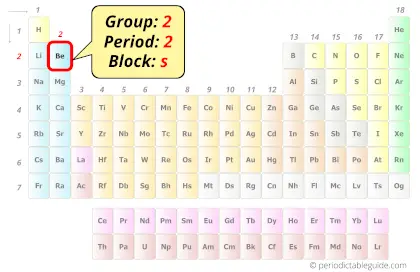 Group: 2, Period: 2, Block: s |
| Category | 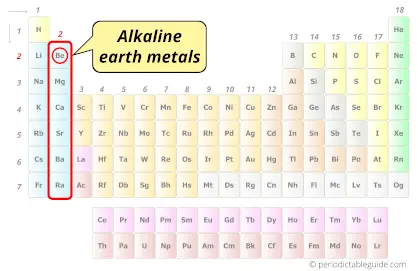 Alkaline earth metals |
| Atomic number or Protons | 4 |
| Neutrons | 5 |
| Electrons | 4 |
| Symbol | Be |
| Atomic mass | 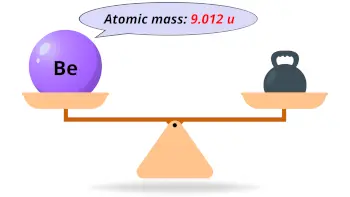 9.012 u |
| Electrons arrangement or Bohr model | 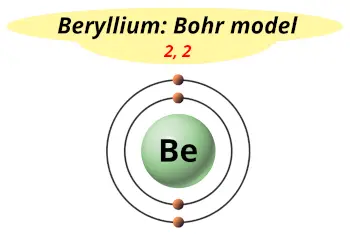 2, 2 |
| Electronic configuration | [He] 2s2 |
| Atomic radius | 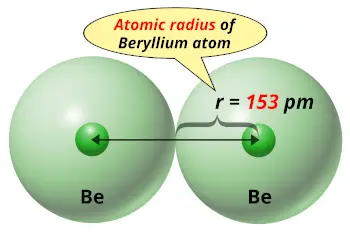 153 picometers (van der Waals radius) |
| Valence electrons | 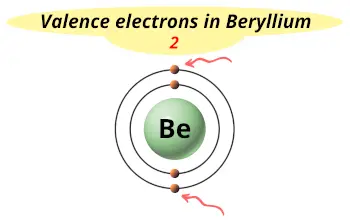 2 |
| 1st Ionization energy | 9.323 eV |
| Electronegativity | 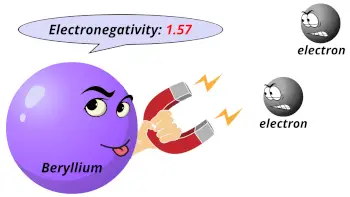 1.57 (Pauling scale) |
| Crystal structure | 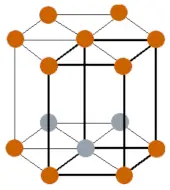 HCP (Hexagonal closed packing) |
| Melting point | 1560 K or 1287 °C or 2349 °F |
| Boiling point | 2742 K or 2469 °C or 4476 °F |
| Density | 1.85 g/cm3 |
| Main isotope | 9Be |
| Who discovered Beryllium and when? |  Nicholas Louis Vauquelin in 1798 |
| CAS number | 7440-41-7 |
Beryllium in Periodic table
Beryllium element is in group 2 and period 2 of the Periodic table. Beryllium is the s-block element and it belongs to alkaline earth metals group.
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba | La* | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| *Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| **Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
←Move to: Lithium (Li) element – Periodic Table
→Move to: Boron (B) element – Periodic Table
Why is Beryllium in Group 2 and Period 2 of the Periodic table?
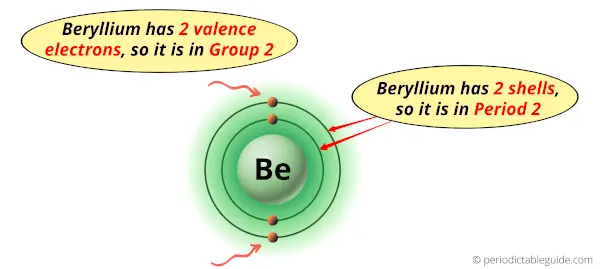
Beryllium is in group 2 because it has 2 valence electrons.
Beryllium is in period 2 because it has 2 shells or orbits.
Now I’ll explain to you the perfect detailed reason why beryllium is in group 2, why it is in period 2 and also why it is in s-block?
Let’s see the reasons one by one.
Why is Beryllium in Group 2?
Do you know, how many electrons can be accommodated in the first shell, second shell, third shell, fourth shell, etc…?
Here is the table showing the capacity of orbits to hold electrons.
Number of electrons in shells.
| Orbit / Shell (n) | Maximum no. of electrons this orbit can hold |
| K shell, n = 1 | 2 × 1² = 2 |
| L shell, n = 2 | 2 × 2² = 8 |
| M shell, n = 3 | 2 × 3² = 18 |
| N shell, n = 4 | 2 × 4² = 32 |
Thus,
- 1st shell can hold 2 electrons.
- 2nd shell can hold 8 electrons.
- 3rd shell can hold 18 electrons.
- 4th shell can hold 32 electrons.
The atomic number of Beryllium (Be) is 4. Hence beryllium element has the electrons arrangement 2, 2. This electron arrangement indicates that the outermost orbit of Beryllium (Be) has 2 electrons. Hence, Beryllium lies in group 2.
Why is Beryllium in Period 2?
Let me ask you a question.
How many shells does Beryllium have?
It’s 2. Right?
You have already seen the Bohr model of Beryllium atom in the above table and you have seen that the number of orbits or shells in sodium is 2.
Hence, as it has 2 orbits, it lies in the 2nd period of the Periodic table.
Why is Beryllium in s-block?
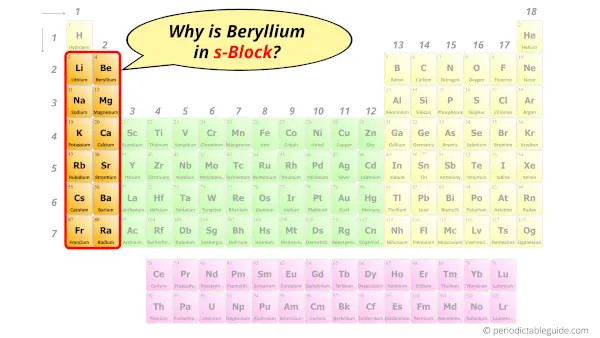
Before knowing this reason, first of all a simple question to you.
How can you determine the blocks wise position of elements?
The simple answer: The elements will lie in the s, p, d or f block will completely depend upon the subshell in which the last electron will enter.
For example; the electron configuration of Beryllium is [He] 2s2.
So the last electron of Beryllium enters the s-subshell or s-orbital.
Hence, beryllium is the s-block element.
Does Beryllium react with water? Why?
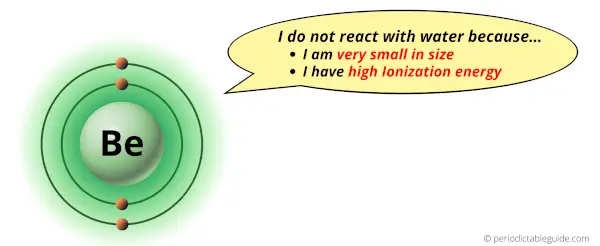
No, Beryllium does not react with water.
There are two reasons why Beryllium does not react with water.
- Small Atomic size
- High ionization energy
Explanation:
Beryllium is the only element in group 2 that does not react with water.
But the question is;
Why it doesn’t show any reaction with water?
Here is the explanation.
1). Small atomic size
The first reason is the smaller atomic size.
Out of all the elements of group 2, the beryllium element has the least atomic size.
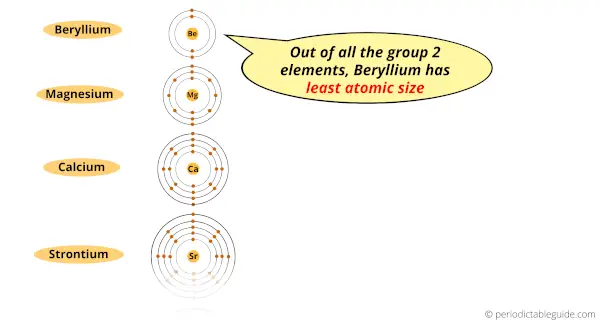
Because of less atomic size of Beryllium, the outermost electrons are highly attracted towards the nucleus.
Because of this high attractive force, beryllium does not lose the outermost electrons during a chemical reaction.
Hence beryllium is not reactive to water.
2). High Ionization energy
The other reason why Beryllium is less reactive is its high ionization energy.
First of all, let me tell you what ionization energy is.
Ionization energy is the minimum energy required to remove the electron/s from the gaseous atom or ion.
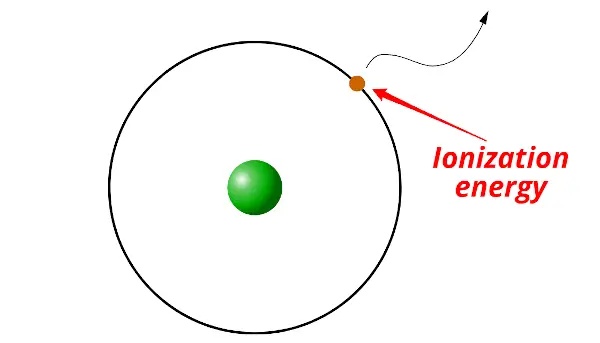
Let me explain this in brief.
The electron of a stable atom cannot escape out of the orbit on its own. It requires some external energy to be supplied on it in order to escape out of the orbit.
This energy which is required to remove the electron is called ionization energy.
Now let’s come back to Beryllium.
Beryllium has very high ionization energy, that means very high amount of energy is required to remove the electron/s of Beryllium.
Hence it becomes difficult for Beryllium atoms to lose electrons.
As the losing of electrons is very difficult for Beryllium, it is not reactive to water.
Also see: Is Lithium reactive to water?
Why is Beryllium less reactive than Magnesium?
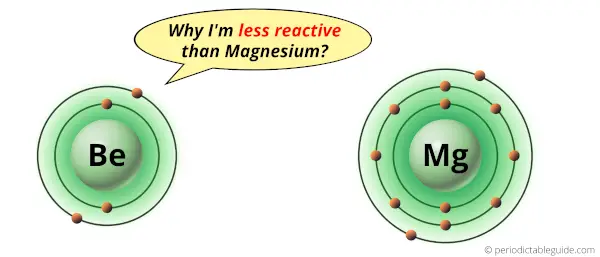
Beryllium and magnesium are the elements of same group on Periodic table (i.e group 2).
But if we compare both of them in terms of reactivity, then Beryllium is less reactive than Magnesium.
The reason why there is a difference in their reactivity is mentioned below.
If we compare the atomic size of Beryllium and magnesium, then the atomic size of Beryllium is very small compared to that of magnesium.
See the Periodic table with atomic size (image) for more information.
Due to the small size of Beryllium, its valence electrons remain highly attracted towards the nucleus of an atom.
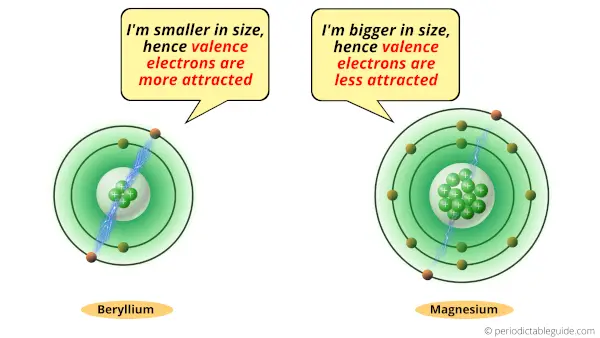
Now if we see the size of magnesium atom, then it is bigger than beryllium atom.
So, the valence electrons are away from the nucleus.
Because of this reason, there is less attractive force between nucleus and valence electrons of magnesium.
So finally, the valence electrons of Beryllium are more attracted towards its nucleus. So it is difficult for it to lose electrons. Hence beryllium is less reactive.
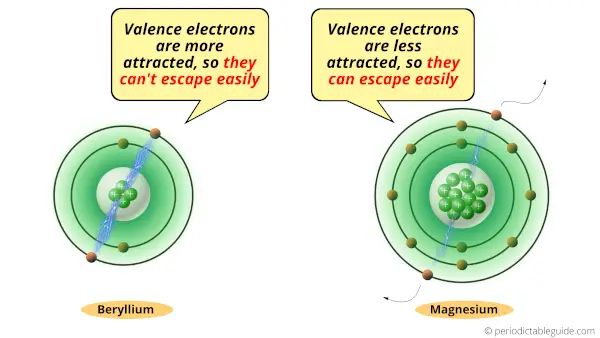
While in magnesium, the valence electrons are far from the nucleus, which means they are less attracted towards the nucleus. So it is easy for magnesium to lose electrons. Hence magnesium is more reactive than Beryllium.
Also due to the difference in their atomic size, their ionization energy also differs.
Beryllium atom is smaller in size. So it has higher Ionization energy.
While magnesium atom is bigger in size. So it has less ionization energy.
Because of higher Ionization energy of Beryllium, it is less reactive than Magnesium.
Why Beryllium is not Alkaline Earth Metal?
First of all you should know what alkaline earth metals are?
Let me give you a very short and simple explanation about alkaline earth metals.
When the group 2 metals (Be, Mg, Ca, Sr, Ba and Ra) react with water, they form hydroxides which are alkaline in nature (or basis in nature).
For example;
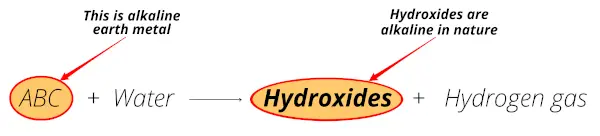
So the above reaction shows that these metals form hydroxides which are alkaline (or basic having pH > 7) in nature.
Plus
These metals are mostly found from the earth crust (in their oxides form) and these oxide minerals are stable to heat.
Hence,
Those metals which fulfills following two criteria:
- On reacting with water, they form hydroxides {Ba(OH)2 , Mg(OH)2 , Ca(OH)2 , etc} which are alkaline in nature and
- Their oxide minerals (BeO, beryl, MgO, magnesite, etc) are mostly found from the earth crust and are stable to heat.
are known as Alkaline Earth Metals.
See the bold word “alkaline” and “earth” in the above 2 points, you will get the exact reason why they are called alkaline earth metals.
Now the question is;
Why is Beryllium (Be) not an alkaline earth metal?
Beryllium (Be) is the only alkaline earth metal which does not form an alkaline solution on reacting with water. Hydroxides of beryllium does not show alkaline behavior, but it shows amphoteric behavior. Hence, beryllium (Be) is not considered as alkaline earth metal.
Why is Beryllium transparent to X-rays?
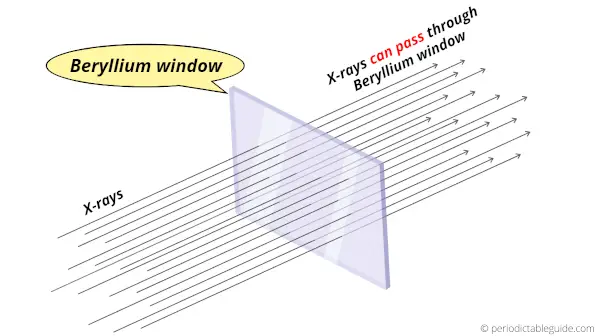
The reasons why the Beryllium window is transparent to X-rays are mentioned below.
- It has only 4 protons and 5 neutrons
- It has 2 core electrons
- Very small Atomic size
- Beryllium has low density (i.e the atoms in Beryllium are far from each other)
Let me give you a simple explanation of the above three points.
Beryllium atom has only 4 protons and 5 neutrons.
This makes the nucleus very small and compact.
The beryllium atom has only 2 core electrons.
Because of very less core electrons, Beryllium can absorb very few X-rays compared to other elements. In other words, Beryllium has low mass absorption coefficient.
Also the beryllium atom has only 2 shells, which indicates that the Atomic size of Beryllium is small.
The density of Beryllium element is very less and this indicates that the atoms of Beryllium are far from each other.
Hence, due to the small number of protons and neutrons, less core electrons, small size of nucleus and more distance between the atoms, the x-rays can easily pass through them.
(Note: If we compare the Beryllium metal and normal glass, then the Beryllium window is more transparent to X-rays than that of the normal glass.
This is because normal glass is made up of silica, which is dense and it has more protons and nucleus, which leads to more absorption of X-rays.)
8 Interesting Facts about Beryllium element
Interesting facts about Beryllium element are mentioned below.
- Beryllium is the lightest alkaline earth metals.
- Beryllium is transparent to X-rays, hence X-rays can easily pass through Beryllium.
- United States, China and Kazakhstan are the only 3 countries that produces beryllium.
- Beryllium is the 44th most abundant element in the earth crust.
- Density of beryllium = ⅔ Density of aluminum
- Beryllium is toxic, so it should never be tasted to know its taste.
- Out of known light metals, beryllium has the highest melting point.
- Pure beryllium is available in very less quantity, but it is mainly obtained from its compounds which are present in the Earth’s crust.
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
External resources:
- Beryllium – Wikipedia. (2007, December 10). Beryllium – Wikipedia. https://en.wikipedia.org/wiki/Beryllium
- Beryllium – Element information, properties and uses | Periodic Table. (n.d.). Beryllium – Element Information, Properties and Uses | Periodic Table. https://www.rsc.org/periodic-table/element/4/beryllium
- P. (n.d.). Beryllium | Be (Element) – PubChem. Beryllium | Be (Element) – PubChem. https://pubchem.ncbi.nlm.nih.gov/element/Beryllium
- Periodic Table of Elements: Los Alamos National Laboratory. (n.d.). Periodic Table of Elements: Los Alamos National Laboratory. https://periodic.lanl.gov/4.shtml
