This is a simple and easy guide on Magnesium element.
In fact, the table mentioned below is the perfect information box (Which gives you every single detail about the Magnesium element in Periodic table.)
So if you want to know anything about Magnesium element, then this guide is for you.
Let’s dive right into it!
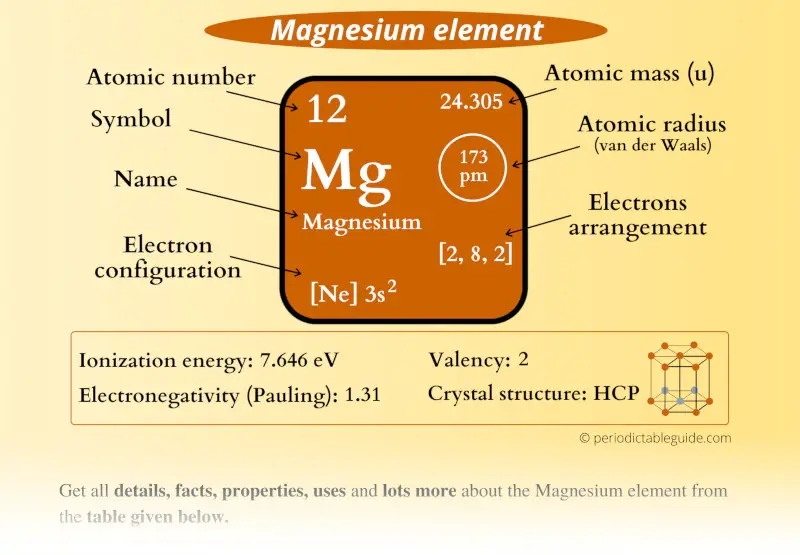
Magnesium Element (Mg) Information
| Appearance |  Shiny grey |
| State (at STP) | Solid |
| Position in Periodic table | 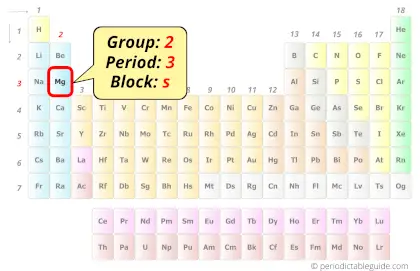 Group: 2, Period: 3, Block: s |
| Category | 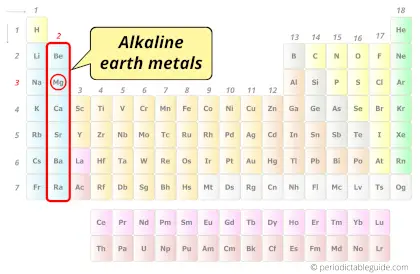 Alkaline earth metals |
| Atomic number or Protons | 12 |
| Neutrons | 12 |
| Electrons | 12 |
| Symbol | Mg |
| Atomic mass |  24.305 u |
| Electrons arrangement or Bohr model | 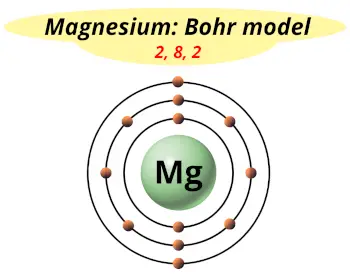 2, 8, 2 |
| Electronic configuration | [Ne] 3s2 |
| Atomic radius | 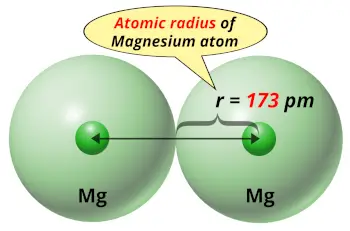 173 picometers (van der Waals radius) |
| Valence electrons | 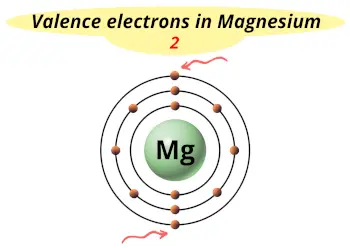 2 |
| 1st Ionization energy | 7.646 eV |
| Electronegativity | 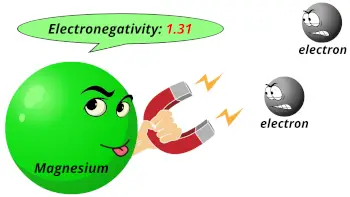 1.31 (Pauling scale) |
| Crystal structure | 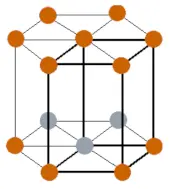 HCP (Hexagonal close packing) |
| Melting point | 923 K or 650 °C or 1202 °F |
| Boiling point | 1363 K or 1091 °C or 1994 °F |
| Density | 1.74 g/cm3 |
| Main isotope | 24Mg |
| Who discovered Magnesium and when? |  Joseph Black in 1755 |
| CAS number | 7439-95-4 |
Magnesium in Periodic table
Magnesium element is in group 2 and period 3 of the Periodic table. Magnesium is the s-block element and it belongs to alkaline earth metals group.
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba | La* | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| *Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| **Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
←Move to: Sodium (Na) element – Periodic Table
→Move to: Aluminum (Al) element – Periodic Table
Why is Magnesium in Group 2?
Do you know, how many electrons can be accommodated in the first shell, second shell, third shell, fourth shell, etc…?
Here is the table showing the capacity of orbits to hold electrons.
Number of electrons in shells.
| Orbit / Shell (n) | Maximum no. of electrons this orbit can hold |
| K shell, n = 1 | 2 × 1² = 2 |
| L shell, n = 2 | 2 × 2² = 8 |
| M shell, n = 3 | 2 × 3² = 18 |
| N shell, n = 4 | 2 × 4² = 32 |
Thus,
- 1st shell can hold 2 electrons.
- 2nd shell can hold 8 electrons.
- 3rd shell can hold 18 electrons.
- 4th shell can hold 32 electrons.
Now the atomic number of Magnesium (Mg) is 12.
Hence the magnesium element has electrons arrangement 2, 8, 2.
This electron arrangement indicates that the outermost orbit of Magnesium element (Mg) has 2 electrons.
Hence, it lies in group 2.
Why is Magnesium in Period 3?
Let me ask you a question.
How many shells does magnesium have?
It’s 3. Right?
You have already seen the bohr model of magnesium element in the above table.
From the Bohr model, it can be found that the number of orbits or shells in magnesium is 3. Hence, as magnesium has 3 orbits, it lies in period 3 of the Periodic table.
Why is Magnesium in s-block?
Before knowing this reason, first of all I want to ask you a simple question.
How can you determine the blocks-wise position of elements?
The simple answer: The elements will lie in the s, p, d or f block will completely depend upon the subshell in which the last electron will enter.
For example; the electron configuration of magnesium is [Ne] 3s2.
So the last electron of magnesium enters the s-subshell or s-orbital.
Hence, magnesium is the s-block element.
7 Interesting facts about Magnesium
Facts about magnesium element are mentioned below.
- Magnesium is the 8th most abundant element in the earth’s crust.
- Magnesium is present in the earth’s crust as well as sea water.
- Magnesium is a word derived from the Greek region “magnesia”.
- Magnesium is important for plants to perform photosynthesis, and the magnesium element is present in the center of the chlorophyll element.
- Magnesium is an essential part of the human body and it is required for biochemical reactions in the body.
- Magnesium is the 11th abundant element (by mass) found in the human body.
- Out of the total amount of magnesium present in the human body, around 60% of magnesium is in the skeleton, 39% is in muscle tissues and 1% being extracellular.
Properties of Magnesium
The physical and chemical properties of magnesium element are mentioned below.
Physical properties of Magnesium
Physical properties of magnesium are mentioned below.
- Magnesium is an alkaline earth metal having a shiny-grey appearance.
- Magnesium is a soft and lightweight metal.
- Density of magnesium is 1.74 g/cm3 which is two-third the density of aluminium.
- Melting point of magnesium is 923 K and its boiling point is 1363 K, which is the lowest of all the alkaline earth metals.
- Ductility of magnesium increases when it is alloyed with 1% aluminum.
Chemical properties of Magnesium
Chemical properties of magnesium are mentioned below.
- Magnesium is chemically reactive metal, and so it always exists in a compound form with other metals in the earth’s crust.
- When magnesium is kept open in air, it will react with the oxygen of the air and form an oxide layer.
- When magnesium is submerged in water, it will produce hydrogen gas. (You will see bubbles forming on the magnesium metal which is submerged in the water.)
- When magnesium is exposed to fire, it forms a bright whikte light.
- Magnesium reacts with most acids like hydrochloric acid and liberates heat during this reaction.
Uses of Magnesium
Uses of magnesium are mentioned below.
- In order to produce a light and strong metal, magnesium is mixed with aluminium in the manufacturing of car bodies, drinking cans, etc.
- Magnesium is also alloyed with metals like zinc, silicon, manganese, etc. to make lightweight alloys.
- Magnesium is a flammable metal and hence it is also used in fireworks and flares.
- Magnesium hydroxide is used in manufacturing fire resistant plastics.
- Human body also requires magnesium as a mineral element for maintaining good health.
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
External resources:
- Magnesium – Element information, properties and uses | Periodic Table. (n.d.). Magnesium – Element Information, Properties and Uses | Periodic Table. https://www.rsc.org/periodic-table/element/12/magnesium
- Magnesium – Wikipedia. (2022, September 1). Magnesium – Wikipedia. https://en.wikipedia.org/wiki/Magnesium
- Cotton, S. (2014, August 12). Magnesium oxide. Magnesium Oxide | Podcast | Chemistry World. https://www.chemistryworld.com/podcasts/magnesium-oxide/7645.article
- P. (n.d.). Magnesium | Mg (Element) – PubChem. Magnesium | Mg (Element) – PubChem. https://pubchem.ncbi.nlm.nih.gov/element/Magnesium
- It’s Elemental – The Element Magnesium. (n.d.). It’s Elemental – the Element Magnesium. https://education.jlab.org/itselemental/ele012.html
