This is the SUPER easy guide on Helium element.
In fact, the table mentioned below is the perfect information box (Which gives you every single detail about the Helium element in Periodic table.)
So if you want to know anything about Helium element, then this guide is for you.
Let’s finish this very quickly.
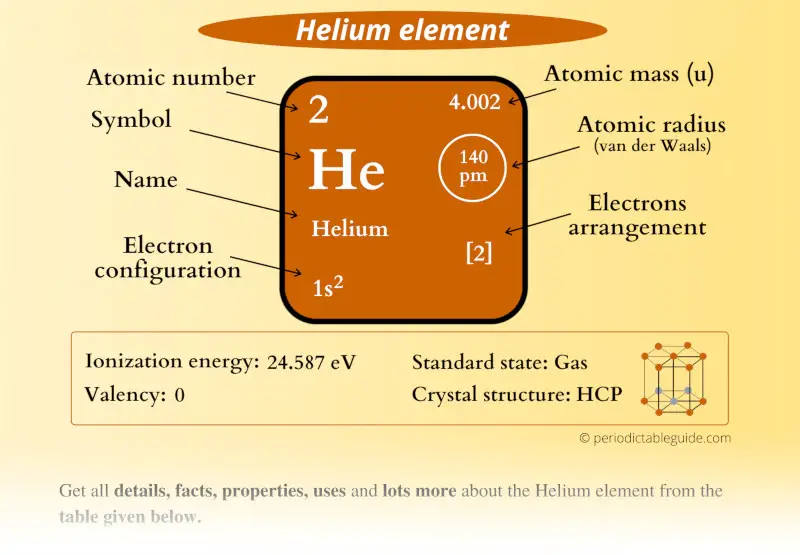
Helium Element (He) Information
| Appearance | Colorless gas |
| State (at STP) | Gas |
| Position in Periodic table | 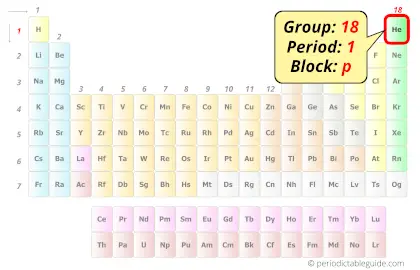 Group: 18, Period: 1, Block: p |
| Category |  Noble gases |
| Atomic number or Protons | 2 |
| Neutrons | 2 |
| Electrons | 2 |
| Symbol | He |
| Atomic mass |  4.002 u |
| Electrons arrangement or Bohr model | 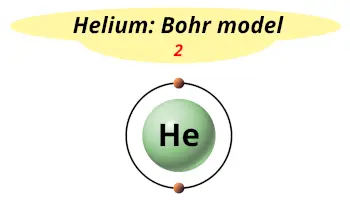 2 electrons in 1st shell |
| Electronic configuration | 1s2 |
| Atomic radius | 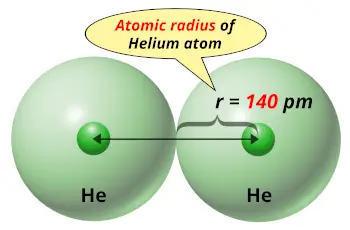 140 picometers (van der Waals radius) |
| Valence electrons | 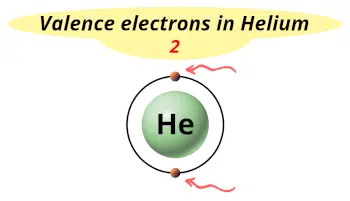 2 |
| 1st Ionization energy | 24.587 eV |
| Crystal structure | 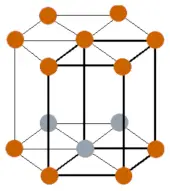 HCP (Hexagonal closed packing) |
| Melting point | 0.95 K or -272.2 °C or -457.9 °F (at 2.5 MPa) |
| Boiling point | 4.22 K or -268.9 °C or -452 °F |
| Density | 0.1786 g/L |
| Main isotope | 4He |
| Who discovered Helium and when? |  1). Pierre Jules César Janssen, and 2). Sir Joseph Norman Lockyer (Discovered in 1868) |
| CAS number | 7440-59-7 |
Helium in Periodic table
Helium element is in period 1 and group 18 of the Periodic table. Helium is the p-block element and it belongs to the Noble gases group.
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba | La* | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| *Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| **Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
←Move to: Hydrogen (H) element – Periodic Table
→Move to: Lithium (Li) element – Periodic Table
Why is Helium not in Group 2?
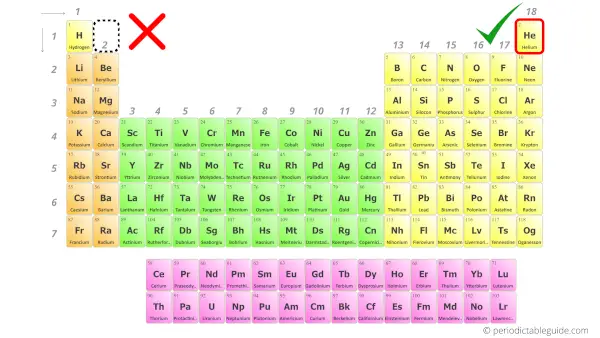
Do you know how many valence electrons helium has?
It has 2 valence electrons.
So it should be placed in group 2 of the Periodic table, but in reality it is not so.
It is placed in group 18 instead of placing it in group 2.
Why?
Let me explain to you the reasons why helium is not in Group 2 and why it is in group 18.
Helium is the element which has 2 electrons only, and these two electrons are nothing but they are the valence electrons.
Helium element is very happy and stable with these two electrons.
(Note: Generally for all the elements, octet is the stable configuration. But helium is the only exception in which duplet configuration is stable.)
Helium shows the very similar physical and chemical properties as that of the group 18 elements.
Helium element do not react with other elements and hence it is chemically inert.
As helium has the same properties (like that of neon, argon, krypton, xenon and radon), it is placed along with these elements (in group 18) and not in group 2.
I hope you have clearly understood the reason why helium is not in Group 2.
Why is Helium in Period 1?
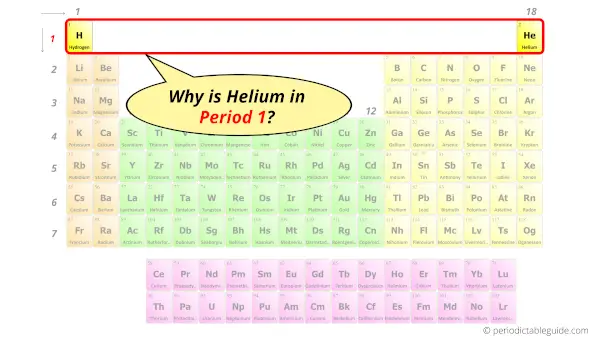
First of all let me ask you a simple question.
How many shells or orbits does the helium element have?
It’s only one, right?
You have already seen the Bohr model of Helium atom in the above table. It has only one shell.
And the number of shells indicates the position of elements in the period.
Hence, as Helium has 1 shell, it lies in period 1.
Why is Helium in p-block?
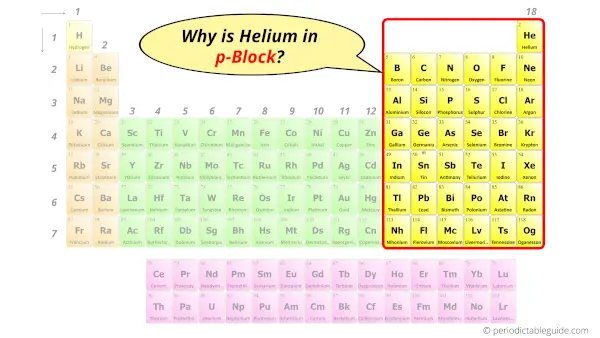
Before knowing this reason, first of all a simple question to you.
How can you determine the blocks wise position of elements?
The simple answer: The elements will lie in the s, p, d or f block will completely depend upon the subshell in which the last electron will enter.
For example; the electron configuration of Helium is 1s2.
So the last electron of helium enters the s-subshell or s-orbital.
Hence, helium should be placed in the s-block of Periodic table.
But it is not so. On the modern periodic table, helium is placed in the p-block instead of s-block.
This is because Helium shows similar properties as that of Noble gases.
As its properties are similar to the Noble gases, it is placed along with them in group 18 in p-block.
Is Helium explosive?

No.
Helium does not explode when it is subjected to fire.
There are many misconceptions regarding the explosion of helium gas.
You might have heard about the explosion of helium gas balloons.
But the fact is that it is not helium gas which is filled in the balloons.
The balloons which catch fire are the hydrogen gas balloons (not the helium gas balloons.)
Helium is a non explosive gas.
Helium is chemically inert gas and so it does not react with any other elements.
But sometimes the explosion of helium tanks may occur.
Why?
The reason is mechanical failure of the tank due to high pressure.
The helium tank is filled in the tank with a high pressure.
The pressure is so high that the helium gas becomes liquid inside the cylinder.
For storing the gas at such high pressure, the tanks must resist the high pressure of the gas.
If the tank is corroded or if it is non resistive to the high pressure, then there are chances of explosion of the cylinder.
Finally, I want to tell you that Helium gas is non reactive and non explosive. That means if you put an ignited matchstick near the pure helium gas, then it will not explode.

But sometimes explosion of helium tank occur due to mechanical failure, and not because of the chemical properties of the helium.
I hope this is now clear to you.
Why is Helium the Smallest atom?
Helium is the smallest atom with the atomic radius 140 picometer (van der Waals radius).
But the question is, why is helium the smallest atom?
You can understand the reason behind the smaller size of helium atom from the following two concepts.
- Periodic trends
- Effective nuclear charge
I’ll explain these two concepts in just few seconds. But first of all you should have some basic knowledge about Trends in Periodic table.
Trends are the changes in properties of elements across the period (from left to right) and down the group (from top to bottom) in the Periodic table.
Let’s come to the main point.
Here we want to know about the size of helium atom.
So the important question is;
What is the atomic size trend in the Periodic table?
The simple answer: Atomic size increases down the group (from top to bottom) and it decreases across the period (from left to right).
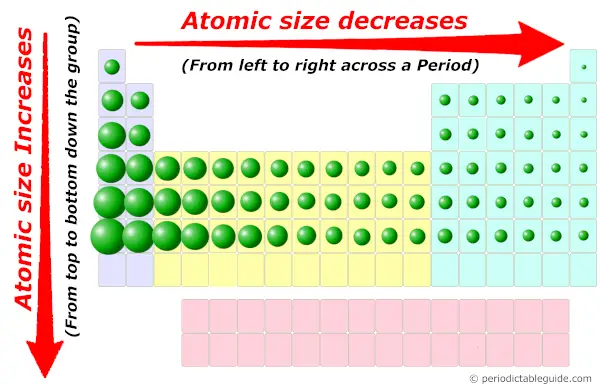
Now the helium atom is located at the far top-right side of the Periodic table.
So according to the Periodic trends, the size of helium atom is the smallest in entire Periodic table.
Now, let me explain the concept in another way.
As we move from left to right in the Periodic table, the effective nuclear charge increases.
Means the number of positively charged protons increases, which attracts the surrounding electrons with a greater force of attraction.
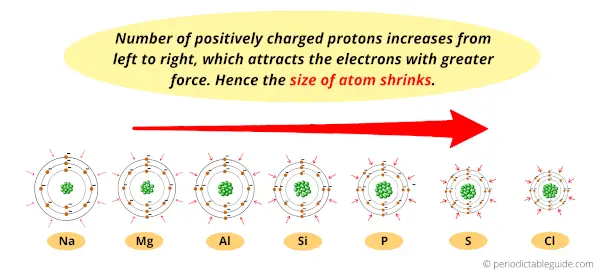
In simple words, the power of nucleus to pull the electrons towards itself will increase.
Now, helium has only one shell and it is also located at the right most column. So it’s electrons will be more attracted towards the nucleus.
Hence due to the attractive force towards the nucleus, the size of helium atoms shrinks.
Hence it is the smallest atom in the Periodic table.
Why is Helium a Noble Gas?
Helium is a noble gas because it has completely filled outermost orbit.
The outermost orbit of helium atom has 2 electrons and it shows a stable duplet configuration.
In other words, the electron configuration of helium element is 1s2.
It has completely filled s-orbitals and it is stable.
Because of this stable configuration, it does not react with any other elements.
Hence, as the helium gas is chemically inert, it is called noble gas.
Also see: List and electronic configuration of Noble gases.
Is Helium Monatomic or Diatomic?

Helium is Monatomic.
Let me explain to you the reason behind this.
Helium has a stable duplet configuration.
In other words, it has two electrons in the outer shell and they are completely stable.
Helium atom do not require any other electrons for its stability.
Due to this stable configuration, the single atoms of helium are highly stable, and so helium exists as monatomic He and not as diatomic He2.
6 Interesting Facts about Helium element
The facts about helium element are mentioned below;
- Helium was first discovered in the Sun’s atmosphere (And not in Earth’s atmosphere.)
- Helium is very very lighter than air. So it always escapes the earth atmosphere and it goes into space.
- At absolute zero temperature, the helium gas becomes liquid and it shows the properties of superfluid.
- Helium is the second most abundant element found in the Universe and it makes roughly up to 24% of it. (Note: Hydrogen is first and it is roughly 74% of all the elements in the universe)
- On the earth, helium is obtained from the decay of radioactive elements like uranium and thorium.
- Helium atom is the smallest atom out of all the known elements.
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
External resources:
- Helium – Element information, properties and uses | Periodic Table. (n.d.). Helium – Element Information, Properties and Uses | Periodic Table. https://www.rsc.org/periodic-table/element/2/helium
- Helium – Wikipedia. (2022, February 1). Helium – Wikipedia. https://en.wikipedia.org/wiki/Helium
- It’s Elemental – The Element Helium. (n.d.). It’s Elemental – the Element Helium. https://education.jlab.org/itselemental/ele002.html
- P. (n.d.). Helium | He (Element) – PubChem. Helium | He (Element) – PubChem. https://pubchem.ncbi.nlm.nih.gov/element/Helium
- Programs: Energy and Minerals: Helium: About Helium | Bureau of Land Management. (n.d.). About Helium | Bureau of Land Management. https://www.blm.gov/programs/energy-and-minerals/helium/about-helium
