This is a SUPER easy guide on Nitrogen element.
In fact, the table mentioned below is the perfect information box (Which gives you every single detail about the nitrogen element in Periodic table.)
So if you want to know anything about Nitrogen element, then this guide is for you.
Let’s finish this very quickly.
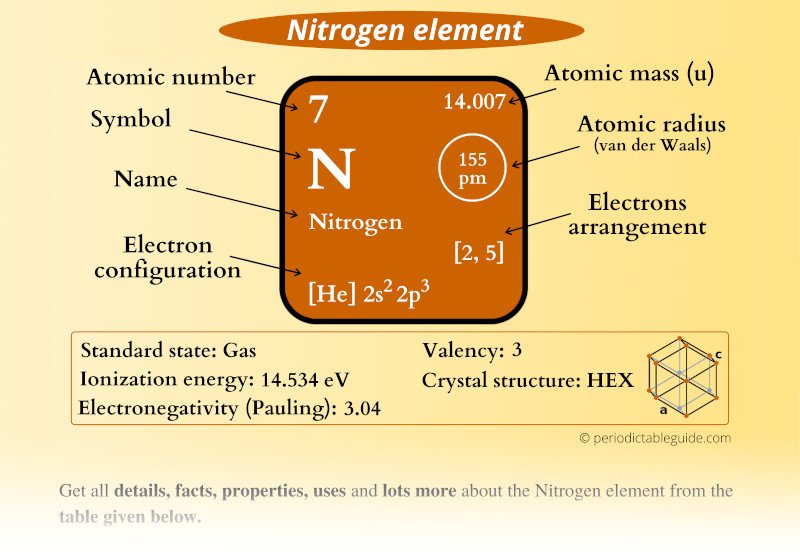
Nitrogen Element (N) Information
| Appearance | Colorless gas |
| State (at STP) | Gas |
| Position in Periodic table | 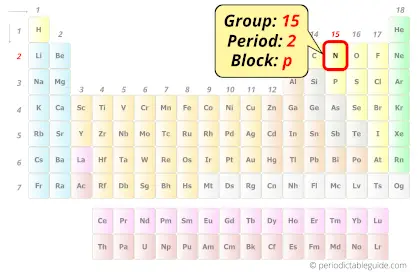 Group: 15, Period: 2, Block: p |
| Category | 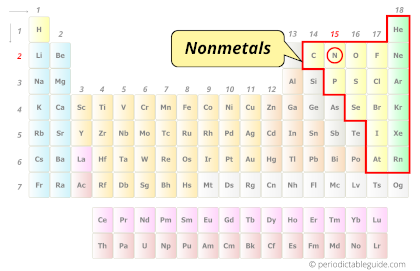 Nonmetals |
| Atomic number or Protons | 7 |
| Neutrons | 7 |
| Electrons | 7 |
| Symbol | N |
| Atomic mass |  14.007 u |
| Electrons arrangement or Bohr model | 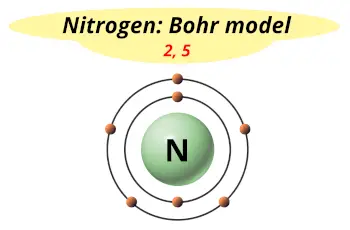 2, 5 |
| Electronic configuration | [He] 2s2 2p3 |
| Atomic radius | 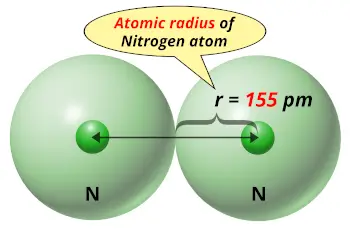 155 picometers (van der Waals radius) |
| Valence electrons | 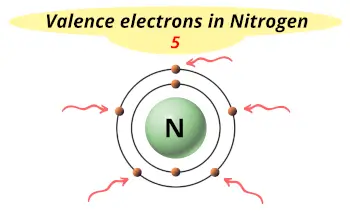 5 |
| 1st Ionization energy | 14.534 eV |
| Electronegativity | 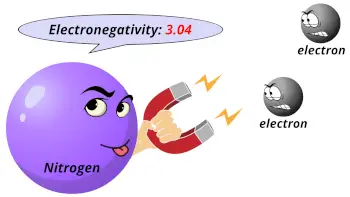 3.04 (Pauling scale) |
| Crystal structure | 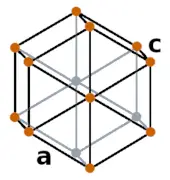 HEX (Hexagonal) |
| Melting point of Nitrogen (N2) | 63.23 K or -209.86 °C or -345.75 °F |
| Boiling point of Nitrogen (N2) | 77.35 K or -195.79 °C or -320.43 °F |
| Density | 0.808 g/cm3 (when liquid at barometric pressure) |
| Main isotope | 14N |
| Who discovered Nitrogen and when? |  Daniel Rutherford (in 1772) |
| CAS number | 7727-37-9 |
Nitrogen in Periodic table
Nitrogen element is in group 15 and period 2 of the Periodic table. Nitrogen is the p-block element and it belongs to the category of Nonmetals.
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba | La* | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| *Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| **Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
←Move to: Carbon (C) element – Periodic Table
→Move to: Oxygen (O) element – Periodic Table
Why is Nitrogen in Group 15 and Period 2 of the Periodic table?
Nitrogen is in group 15 because it has 5 valence electrons.
Nitrogen is in period 2 because it has 2 shells or orbits.
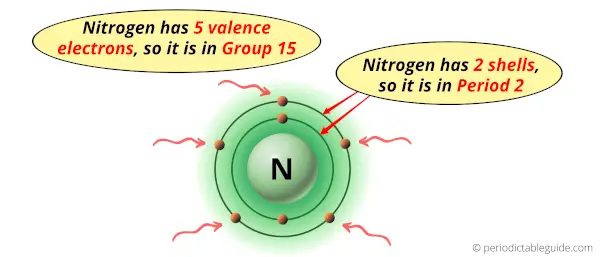
Now I’ll explain to you the perfect detailed reason why nitrogen is in group 15, why it is in period 2 and also why it is in p-block?
Let’s see the reasons one by one.
Why is Nitrogen in Group 15?
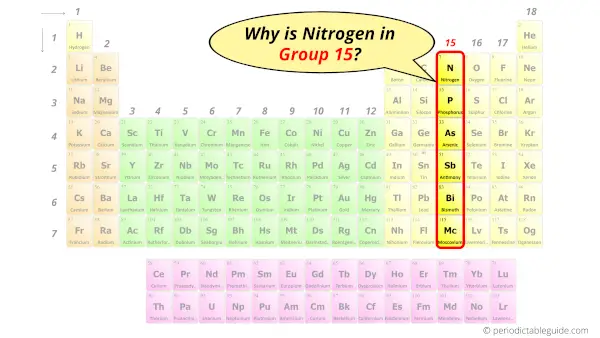
Do you know, how many electrons can be accommodated in the first shell, second shell, third shell, fourth shell, etc…?
Here is the table showing the capacity of orbits to hold electrons.
Number of electrons in shells.
| Orbit / Shell (n) | Maximum no. of electrons this orbit can hold |
| K shell, n = 1 | 2 × 1² = 2 |
| L shell, n = 2 | 2 × 2² = 8 |
| M shell, n = 3 | 2 × 3² = 18 |
| N shell, n = 4 | 2 × 4² = 32 |
Thus,
- 1st shell can hold 2 electrons.
- 2nd shell can hold 8 electrons.
- 3rd shell can hold 18 electrons.
- 4th shell can hold 32 electrons.
Now the atomic number of Nitrogen (N) is 7.
Hence nitrogen element has the electrons arrangement 2, 5.
This electron arrangement indicates that the outermost orbit of Nitrogen (N) has 5 electrons.
Hence, it lies in group 15.
Why is Nitrogen in Period 2?
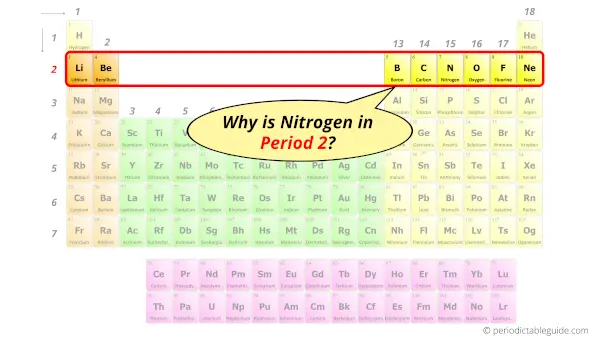
Let me ask you a question.
How many shells does Nitrogen have?
It’s 2. Right?
You have already seen the bohr model of nitrogen element in the above table.
From the Bohr model, it can be found that the number of orbits or shells in nitrogen is 2.
Hence, as nitrogen has 2 orbits, it lies in period 2 of the Periodic table.
Why is Nitrogen in p-block?
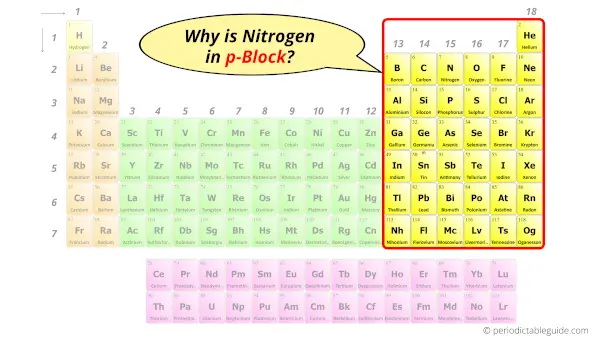
Before knowing this reason, first of all a simple question to you.
How can you determine the blocks wise position of elements?
The simple answer: The elements will lie in the s, p, d or f block will completely depend upon the subshell in which the last electron will enter.
For example; the electron configuration of nitrogen is [He] 2s2 2p3.
So the last electron of Nitrogen enters the p-subshell or p-orbital.
Hence, nitrogen is the p-block element.
Why is Nitrogen an important element for sustaining life?
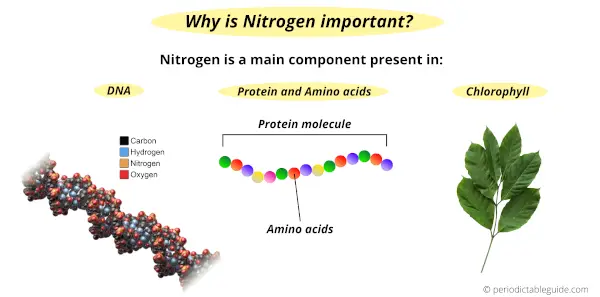
Nitrogen element is very important for sustaining life because of the following reasons.
- Nitrogen is present in the nucleic acid of our body which includes DNA and RNA. DNA is responsible for the transfer of genetic information from one generation to another.
- Also nitrogen is a part of amino acids, which are the building block of proteins in our body.
Our skin, hair, muscles, nails, blood, etc contains proteins. For their growth, replacement or repair, nitrogen is very essential. - The nitrogen present in human body does not enter directly from the air, but it enters our body from the food which we eat.
- Apart from humans, plants also need nitrogen for their growth. Plants prepare their food during the photosynthesis process. Chlorophyll which is responsible for the green color of the plants has a large amount of nitrogen in it.
- Plants receive nitrogen from the water and soil in the form of nitrates and ammonium.
Plants which do not get enough nitrogen turn yellow and they also lack growth. Also too much nitrogen is also harmful for the growth of plants. - As nitrogen is important for plants, there are many nitrogen-containing fertilizers available for the better growth of plants.
Thus nitrogen is very important for plants and animals to grow, survive and reproduce.
Why is Nitrogen 78 percent of the atmosphere?

There are few reasons which make 78% of Nitrogen in the atmosphere.
- Nitrogen gas does not get easily involved in chemical reaction with other elements present in the Earth’s atmosphere.
- Also Nitrogen and oxygen are heavy compared to other gases present in the atmosphere. Hence they remain close to Earth’s surface. Other gases like hydrogen, helium, argon, neon, etc are lighter than nitrogen. So they tend to remain at the top layer of atmosphere.
Because of these reasons, nitrogen is 78 % in Earth’s atmosphere.
Is Nitrogen lighter or heavier than air?
Nitrogen is 3% lighter than air.
However, this value is very small and the reason behind such a small value is the difference in atomic mass of Nitrogen and oxygen.
Let me tell you the atomic mass of Nitrogen and oxygen.
- Atomic mass of Nitrogen element is 14.007 u,
- Atomic mass of Oxygen element is 15.999 u.
Hence, nitrogen is lighter than air (oxygen).
But the question is;
If nitrogen is lighter than oxygen, then why shouldn’t all nitrogen go up in the atmosphere and all oxygen settles at the bottom of the Earth’s surface?

Interesting question, isn’t it?
Now read this answer very carefully.
Nitrogen and Oxygen are gases, and gas molecules move a lot (they have a lot of randomness/molecular movement).
Also they show the property of diffusion.
That means Nitrogen and Oxygen mixes very well with each other.
The difference of their masses is very tiny in comparison to their ability to mix with each other.
Also the atmosphere around the earth is constantly changing (like temperature changes, changes in air movement, minor pressure changes, etc)
Due to these changes in temperature & pressure as well as movement of atmosphere, the gases do not remain settled at one place.
Also the mixing of both Nitrogen and Oxygen is a spontaneous process which leads to increase in entropy of the universe.
Hence, even if Nitrogen gas is lighter than air, it does not move upward in the earth’s atmosphere.
Is Nitrogen diatomic or monatomic? Why?

Nitrogen is Diatomic.
Let me explain to you the reason behind this.
Nitrogen atom has 5 electrons in its outermost orbit.
Now you might be knowing that having 5 electrons in the outermost orbit is not a stable configuration.
The atom should have duplet or octet configuration for its stability.
Nitrogen has 5 electrons in its outermost orbit and it needs 3 more electrons to complete the octet configuration.
Thus the nitrogen atom combines with the other nitrogen atom by sharing 3 electrons. In this way, it forms three covalent bonds which is also known as triple bond.
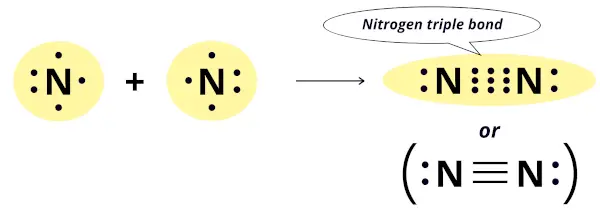
Out of these three covalent bonds, one is a sigma bond while the other two are pi-bonds.
Hence, nitrogen exists as a diatomic molecule.
Also read:
1). Why is helium monatomic?
2). Why is hydrogen diatomic?
Is Nitrogen necessary for breathing?
We only need oxygen for our body.
Let me explain in simple words:
The air which we breathe in contains 78% of nitrogen and 21% oxygen.

So, right now you are breathing 78% of nitrogen as well as 21% of oxygen gas.
But the fact is that our body needs only oxygen for proper functioning of our body.
Nitrogen is not necessary for our body.
So right now when you are exhaling out, you are removing that 78% of nitrogen.
Our body uses the portion of oxygen gas for metabolic activities and releases the carbon dioxide gas when we breathe out.
Hence, nitrogen is useless gas which enters our body and comes out from the body, without creating any harm to our body.
(Note: Nitrogen gas is nonreactive gas and it is safe to breathe only when it is mixed with a proper amount of oxygen. But breathing pure nitrogen gas is harmful.)
7 Interesting facts about Nitrogen element
Interesting facts about Nitrogen element are mentioned below:
- The air we breathe contains around 78% of Nitrogen.
- Nitrogen is present in all the living organisms (including humans, plants and animals).
- Around 3 % of our body mass is due to nitrogen. Nucleic acid (DNA & RNA), proteins, etc contain nitrogen element in it.
- Titan (which is the largest moon of Saturn) has around 98% of nitrogen in its atmosphere.
- Nitrogen gas makes up around 78% of the Earth’s atmosphere.
- Liquid nitrogen is so cold that it burns the skin and causes severe damage.
- Nitrogen is the 7th most abundant element in the universe.
Also see:
1). 10 Interesting facts about hydrogen
2). 6 Interesting facts about helium
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
External resources:
- Nitrogen – Element information, properties and uses | Periodic Table. (n.d.). Nitrogen – Element Information, Properties and Uses | Periodic Table. https://www.rsc.org/periodic-table/element/7/nitrogen
- Nitrogen | Definition, Symbol, Uses, Properties, Atomic Number, & Facts. (n.d.). Encyclopedia Britannica. https://www.britannica.com/science/nitrogen
- P. (n.d.). Nitrogen | N (Element) – PubChem. Nitrogen | N (Element) – PubChem. https://pubchem.ncbi.nlm.nih.gov/element/Nitrogen
- It’s Elemental – The Element Nitrogen. (n.d.). It’s Elemental – the Element Nitrogen. https://education.jlab.org/itselemental/ele007.html
- Periodic Table of Elements: Los Alamos National Laboratory. (n.d.). Periodic Table of Elements: Los Alamos National Laboratory. https://periodic.lanl.gov/7.shtml
