This is a SUPER easy guide on Gallium element.
In fact, the table mentioned below is the perfect information box (Which gives you every single detail about the Gallium element in Periodic table.)
So if you want to know anything about Gallium element, then this guide is for you.
Let’s dive right into it!
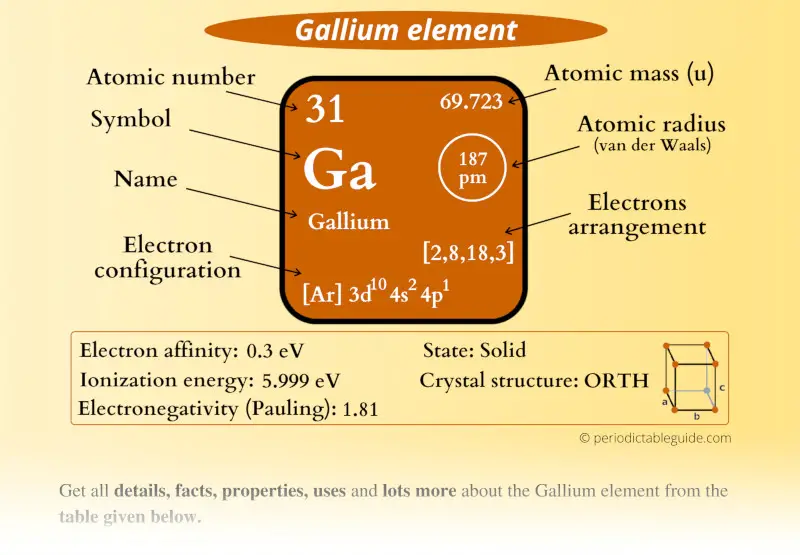
Gallium Element (Ga) Information
| Appearance |  Silvery blue in color |
| State (at STP) | Solid |
| Position in Periodic table | 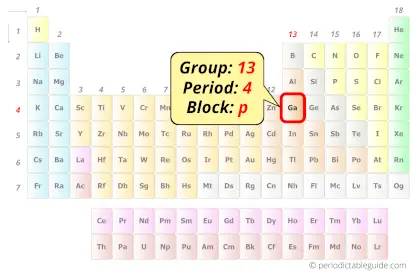 Group: 13, Period: 4, Block: p |
| Category | 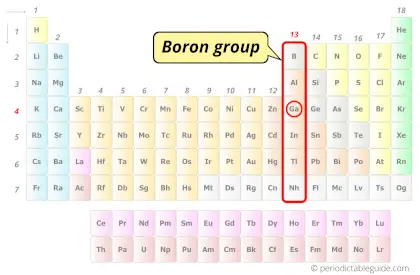 Boron group |
| Atomic number or Protons | 31 |
| Neutrons | 39 |
| Electrons | 31 |
| Symbol | Ga |
| Atomic mass | 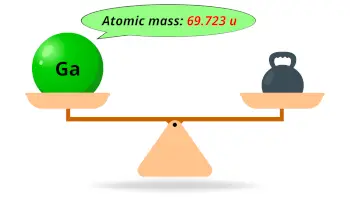 69.723 u |
| Electrons arrangement or Bohr model | 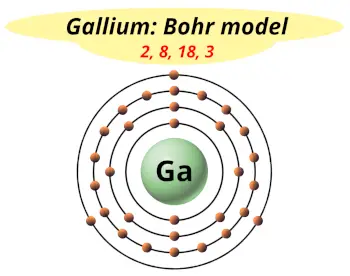 2, 8, 18, 3 |
| Electronic configuration | [Ar] 3d10 4s2 4p1 |
| Atomic radius | 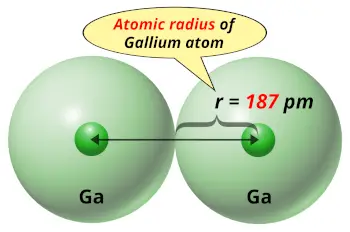 187 picometers (van der Waals radius) |
| Valence electrons | 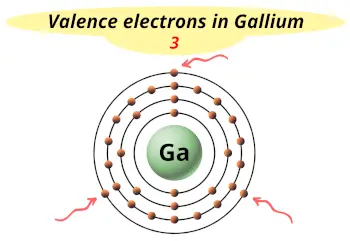 3 |
| 1st Ionization energy | 5.999 eV |
| Electronegativity | 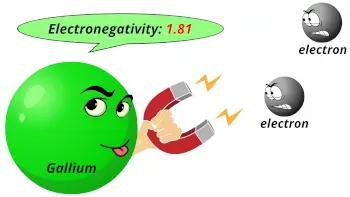 1.81 (Pauling scale) |
| Crystal structure |  Orthorhombic |
| Melting point | 302.9 K or 29.7 °C or 85.5 °F |
| Boiling point | 2673 K or 2400 °C or 4352 °F |
| Density | 5.904 g/cm3 |
| Main isotope | 69Ga |
| Who discovered Gallium and when? |  Lecoq de Boisbaudran in 1875 |
| CAS number | 7440-55-3 |
Gallium in Periodic table
Gallium element is in group 13 and period 4 of the Periodic table. Gallium is the p-block element and it belongs to Boron group.
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba | La* | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| *Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| **Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
←Move to: Zinc (Zn) element – Periodic Table
→Move to: Germanium (Ge) element – Periodic Table
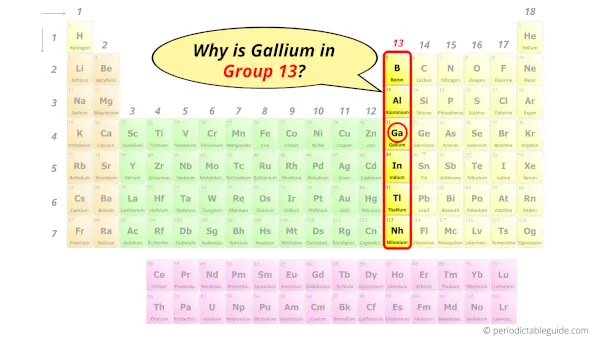
Why is Gallium in Group 13?
Do you know, how many electrons can be accommodated in the first shell, second shell, third shell, fourth shell, etc…?
Here is the table showing the capacity of orbits to hold electrons.
Number of electrons in shells.
| Orbit / Shell (n) | Maximum no. of electrons this orbit can hold |
| K shell, n = 1 | 2 × 1² = 2 |
| L shell, n = 2 | 2 × 2² = 8 |
| M shell, n = 3 | 2 × 3² = 18 |
| N shell, n = 4 | 2 × 4² = 32 |
Thus,
- 1st shell can hold 2 electrons.
- 2nd shell can hold 8 electrons.
- 3rd shell can hold 18 electrons.
- 4th shell can hold 32 electrons.
Now the atomic number of gallium (Ga) is 31.
Hence the gallium element has electrons arrangement 2, 8, 18, 3.
This electron arrangement indicates that the outermost orbit of Gallium element (Ga) has 3 electrons.
Hence, it lies in group 13.
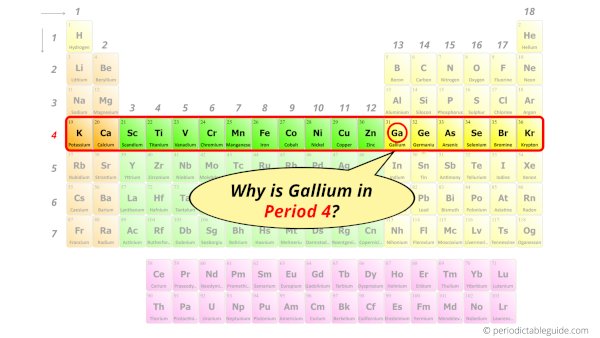
Why is Gallium in Period 4?
Let me ask you a question.
How many shells does gallium have?
It’s 4. Right?
You have already seen the bohr model of gallium atom in the above table.
From the Bohr model, it can be found that the number of orbits or shells in gallium is 4. Hence, as gallium has 4 orbits, it lies in period 4 of the Periodic table.
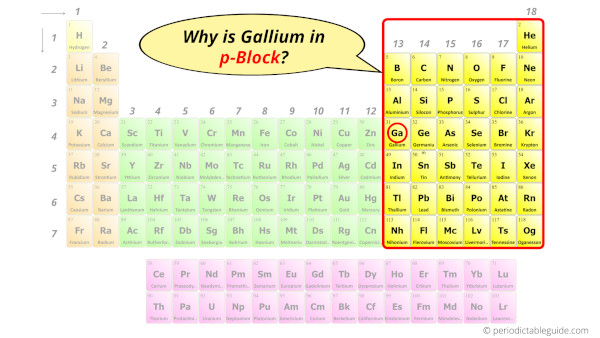
Why is Gallium in p-block?
Before knowing this reason, first of all I want to ask you a simple question.
How can you determine the blocks-wise position of elements?
The simple answer: The elements will lie in the s, p, d or f block will completely depend upon the subshell in which the last electron will enter.
For example; the electron configuration of gallium is [Ar] 3d10 4s2 4p1.
So the last electron of gallium enters the p-subshell or p-orbital.
Hence, gallium is the p-block element.
7 Interesting facts about Gallium
Interesting facts about gallium element are mentioned below.
- The name gallium was derived from the latin word “gallia”.
- Earth’s crust contains around 0.0019% of gallium by weight.
- Gallium is the 34th abundant element found from the earth’s crust.
- Gallium is a soft metal that can be cut even with a kitchen knife.
- The difference between the melting point and boiling point of gallium is high. Its melting point is just very close to room temperature (i.e 29.7 °C), while its boiling point is much higher (i.e 2400 °C).
- If you hold a gallium in your hand, then it will melt down due to the warmth of your hand.
- Gallium expands on freezing. Hence it should not be stored in a glass container.
Properties of Gallium
The physical and chemical properties of gallium element are mentioned below.
Physical properties of Gallium
Physical properties of gallium are mentioned below.
- Gallium is a soft metal having a silvery blue color.
- The melting point of gallium is 29.7 °C and its boiling point is 2400 °C.
- Gallium becomes brittle solid at lower temperatures.
- Gallium has many isotopes, but out of these isotopes, 69Ga is the most abundant (around 60%).
- Gallium expands on freezing.
Chemical properties of Gallium
Chemical properties of gallium are mentioned below.
- At room temperature, gallium is not so reactive as it forms a protective oxide layer on it.
- Gallium reacts with chalcogens elements only at higher temperatures.
- At higher temperatures, gallium also reacts with oxygen to form gallium oxide.
- Also pure gallium is resistive to the attack of mineral acids, because the oxide layer formed on the outer surface of gallium protects the inner metal.
- Gallium is classified as a post transition element on the periodic table as it shows some properties of transition metals as well as it also shows some properties of nonmetals.
Uses of Gallium
Uses of gallium are mentioned below.
- Gallium is generally used in electr0nics.
- Gallium arsenide is a compound that is mostly used in microwave as well as infrared circuits.
- Gallium arsenide is also used to produce laser light by passing electricity through it.
- Gallium nitride is used in blue-ray technology as well as pressure sensors.
- As gallium has a lower melting point, it is also used to manufacture alloys that have lower melting point.
- Gallium metal is also used in high temperature thermometers, barometers and other heat transfer systems.
- Gallium nitride is also used in treatment of hypercalcemia (which is a disease that is responsible for the growth of bone tumors).
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
External resources:
- Gallium – Element information, properties and uses | Periodic Table. (n.d.). Gallium – Element Information, Properties and Uses | Periodic Table. https://www.rsc.org/periodic-table/element/31/gallium
- Gallium – Wikipedia. (2009, July 7). Gallium – Wikipedia. https://en.wikipedia.org/wiki/Gallium
- It’s Elemental – The Element Gallium. (n.d.). It’s Elemental – the Element Gallium. https://education.jlab.org/itselemental/ele031.html
- P. (n.d.). Gallium | Ga (Element) – PubChem. Gallium | Ga (Element) – PubChem. https://pubchem.ncbi.nlm.nih.gov/element/Gallium
