This is a SUPER easy guide on Boron element.
In fact, the table mentioned below is the perfect information box (Which gives you every single detail about the Boron element in Periodic table.)
So if you want to know anything about Boron element, then this guide is for you.
Let’s finish this very quickly.
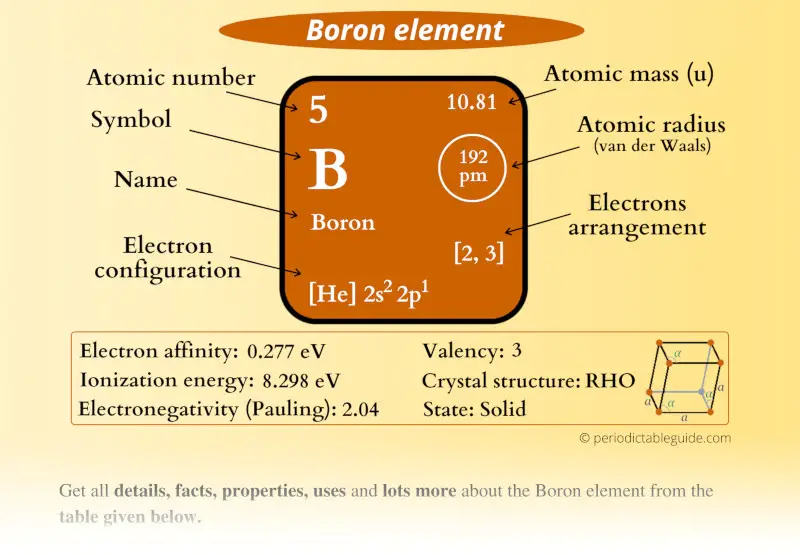
Boron Element (B) Information
| Appearance |  Black and brown |
| State (at STP) | Solid |
| Position in Periodic table | 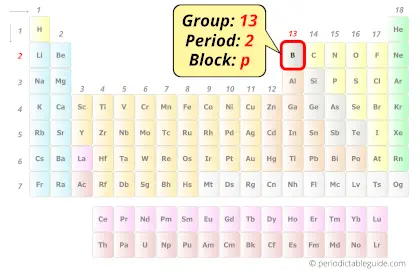 Group: 13, Period: 2, Block: p |
| Category | 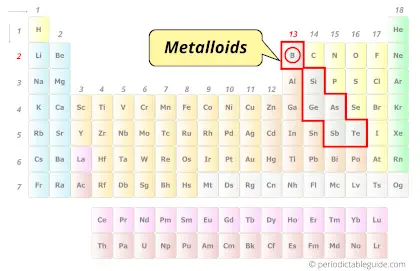 Metalloids |
| Atomic number or Protons | 5 |
| Neutrons | 6 |
| Electrons | 5 |
| Symbol | B |
| Atomic mass |  10.81 u |
| Electrons arrangement or Bohr model | 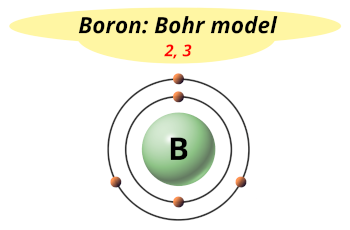 2, 3 |
| Electronic configuration | [He] 2s2 2p1 |
| Atomic radius | 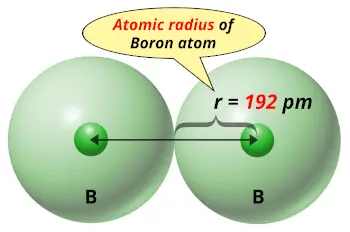 192 picometers (van der Waals radius) |
| Valence electrons | 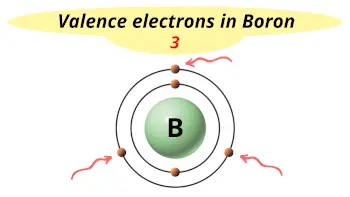 3 |
| 1st Ionization energy | 8.298 eV |
| Electronegativity | 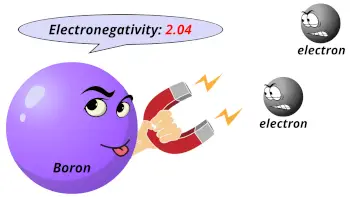 2.04 (Pauling scale) |
| Crystal structure | 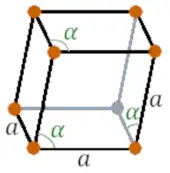 RHO (Rhombohedral) |
| Melting point | 2349 K or 2076 °C or 3769 °F |
| Boiling point | 4200 K or 3927 °C or 7101 °F |
| Density | 2.08 g/cm3 |
| Main isotope | 11B |
| Who discovered Boron and when? |  1). Joseph Louis Gay-Lussac and 2). Louis Jacques Thenard (Discovered in 1808) |
| CAS number | 7440-42-8 |
Boron in Periodic table
Boron element is in group 13 and period 2 of the Periodic table. Boron is the p-block element and it is a metalloid.
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba | La* | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| *Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| **Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
←Move to: Beryllium (Be) element – Periodic Table
→Move to: Carbon (C) element – Periodic Table
Why is Boron in Group 13 and Period 2 of the Periodic table?
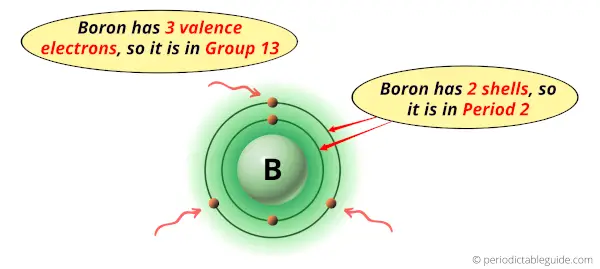
Boron is in group 13 because it has 3 valence electrons.
Boron is in period 2 because it has 2 shells or orbits.
Now I will explain to you the perfect reason why boron is in group 13, why it is in period 2 and also why it is in p-block?
Let’s see the reasons one by one.
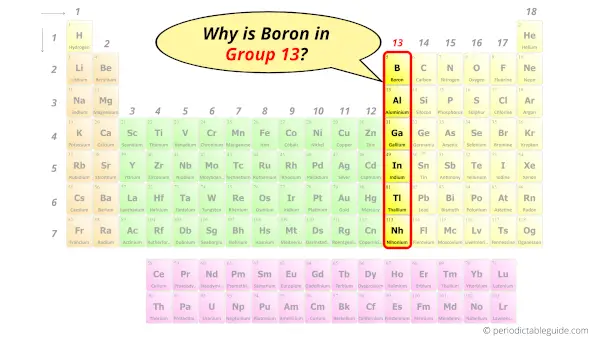
Why is Boron in Group 13?
Do you know, how many electrons can be accommodated in the first shell, second shell, third shell, fourth shell, etc…?
Here is the table showing the capacity of orbits to hold electrons.
Number of electrons in shells.
| Orbit / Shell (n) | Maximum no. of electrons this orbit can hold |
| K shell, n = 1 | 2 × 1² = 2 |
| L shell, n = 2 | 2 × 2² = 8 |
| M shell, n = 3 | 2 × 3² = 18 |
| N shell, n = 4 | 2 × 4² = 32 |
Thus,
- 1st shell can hold 2 electrons.
- 2nd shell can hold 8 electrons.
- 3rd shell can hold 18 electrons.
- 4th shell can hold 32 electrons.
Now the atomic number of Boron (B) is 5.
Hence, boron element has the electrons arrangement 2, 3.
This electron arrangement indicates that the outermost orbit of Boron (B) has 3 electrons.
Hence, it lies in group 13.
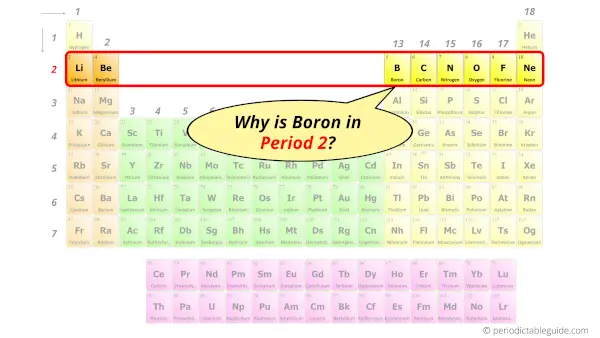
Why is Boron in Period 2?
Let me ask you a question.
How many shells does boron have?
It’s 2. Right?
You have already seen this in the bohr model of boron element in above table.
From the Bohr model, it can be found that the number of orbits or shells in boron atom is 2.
Hence, as boron has 2 orbits, it lies in period 2 of the Periodic table.
Also see: Periodic table with electrons per shell (Image)
Why is Boron in p-block?
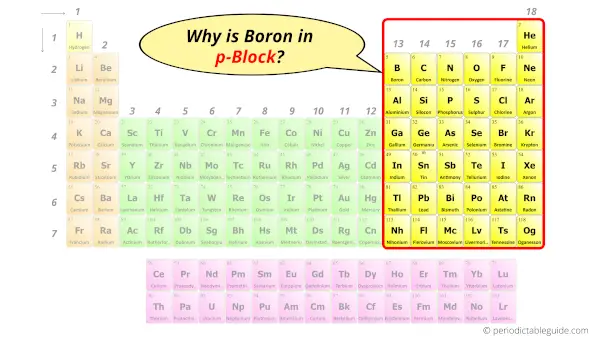
Before knowing this reason, first of all a simple question to you.
How can you determine the blocks wise position of elements?
The simple answer: The elements will lie in the s, p, d or f block will completely depend upon the subshell in which the last electron will enter.
For example;
The electron configuration of Boron is [He] 2s2 2p1.
So the last electron of Boron enters the p-subshell or p-orbital.
Hence, boron is the p-block element.
Why is Boron a Metalloid?

Boron is a Metalloid because boron shows some properties of metals as well as some properties of solid nonmetals.
For example,
Boron shows the properties of Nonmetals when it reacts with elements like sodium (Na), potassium (K), etc.
And boron shows properties of Metals when it reacts with elements like fluorine (F), chlorine (Cl), etc.
Also, boron has a property to form hydrides like B2H6 (which is similar to the hydrides formed by Nonmetals like CH4, PH3, etc).
Boron also shows some physical properties which are similar to that of the metals and solid nonmetals.
Thus the properties of Boron are similar to metals as well as Nonmetals.
This is the reason why boron is classified as a Metalloid.
Why does boron burn green?
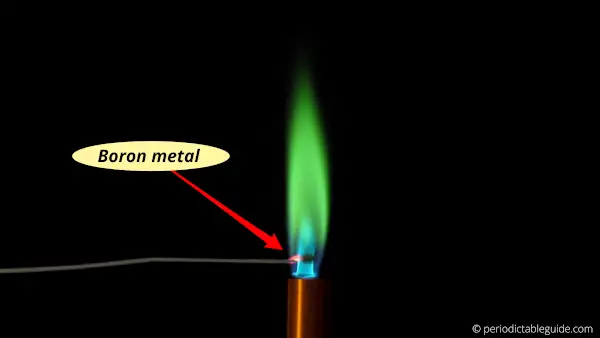
When Boron metal is heated in a flame, its outermost electron gets excited onto a higher energy level.
When this excited electron loses its energy, it again comes back to the lower energy level.
During this phenomenon, it emits an electromagnetic radiation having a wavelength of around 550 nm.
This emitted radiation of 550 nm is nothing but a visible green light.
Hence, lithium gives green flame when it is heated.
Why Boron has high Melting point?

Boron has a high melting point because;
- It has small atomic size
- It has strong covalent bonds with adjacent atoms
- Boron atoms are tightly packed in its solid state
Let me explain these reasons in brief.
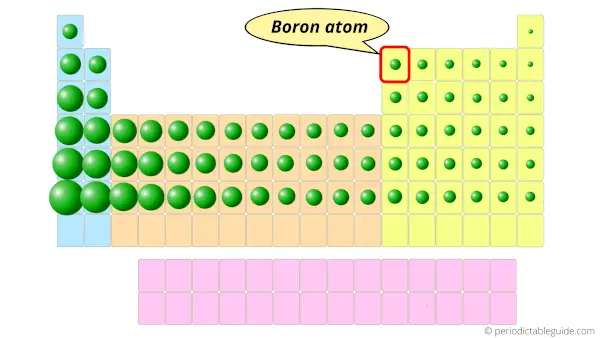
From the above image you can easily see how small the boron atom is !!
If we compare the solid elements on the Periodic table, then boron has very small atomic size.
Because of the smaller atomic size, the boron atoms are closely bonded with each other.
They are tightly attached with each other in their solid state, by forming a strong covalent bond.
Because of the strong covalent bond between the boron atoms, a high amount of heat is required to break these covalent bonds.
Hence, boron has high melting point as well as boiling point.
Why is Boron bigger than Carbon?
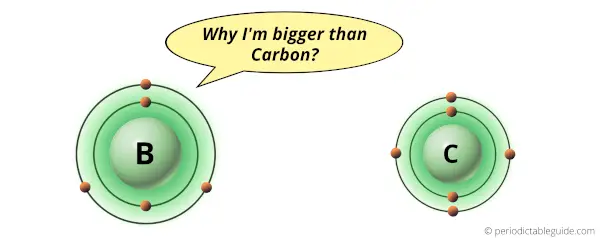
Boron and carbon are the elements in the same period (i.e period 2).
But there is a slight difference in their atomic size. Boron atom is slightly bigger in size than the carbon atom.
Here is the reason behind this.
You might be knowing about the atomic size trend in Periodic table.
According to the atomic size trend, as we move across a period (from left to right in the Periodic table), the atomic size of the elements decreases.
This is because of the increase in attractive force between the positively charged nucleus and negativity charges electrons.
Let me explain this with an example.
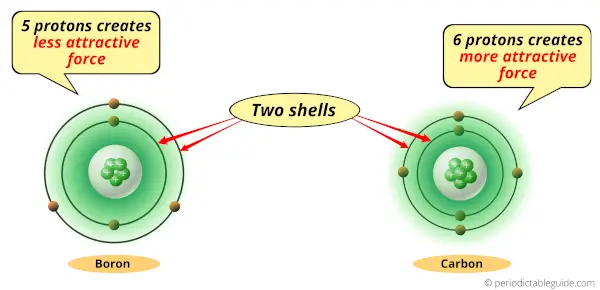
As you can see, Boron and carbon atoms have 2 shells or orbits.
But the difference is the number of protons present in their nucleus.
Boron atom has 5 protons while the carbon atom has 6 protons.
Protons are the positively charged particles which attract the negatively charged electrons.
Now, the Carbon atom has 6 protons, so it generates more attractive force compared to that of Boron.
So if we compare carbon atom and boron atom, then they have same number of shells but carbon atom has more positive charge which attracts the electrons towards the nucleus.
Thus, because of this attractive force, the carbon atom shrinks.
Hence boron is bigger than carbon.
Also see: Periodic table with atomic size labeled on it (Image)
5 Interesting facts about boron element
5 facts about boron element are given below.
- Out of all the metalloids, boron has the highest melting point and boiling point.
- Boron can absorb neutrons, hence it is used in nuclear reactors to control the nuclear reactions.
- Boron burns with a green flame when it is heated.
- Turkey is the largest producer of boron and USA is the second largest producer of boron in the world.
- Out of total available boron, 50% of boron is used in fiberglass and structural materials.
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
External resources:
- Periodic Table of Elements: Los Alamos National Laboratory. (n.d.). Periodic Table of Elements: Los Alamos National Laboratory. https://periodic.lanl.gov/5.shtml
- Chemistry of Boron (Z=5). (2013, October 2). Chemistry LibreTexts. https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Supplemental_Modules_and_Websites_(Inorganic_Chemistry)/Descriptive_Chemistry/Elements_Organized_by_Block/2_p-Block_Elements/Group_13%3A_The_Boron_Family/Z005_Chemistry_of_Boron_(Z5)
- Boron – Wikipedia. (2011, December 4). Boron – Wikipedia. https://en.wikipedia.org/wiki/Boron
- It’s Elemental – The Element Boron. (n.d.). It’s Elemental – the Element Boron. https://education.jlab.org/itselemental/ele005.html
