Atomic mass of all elements (along with the rounded off values) is mentioned in the chart below.
| Atomic Number | Element | Atomic Mass (u) | Atomic mass (u)(Rounded off) |
|---|---|---|---|
| 1 | Atomic mass of Hydrogen (H) | 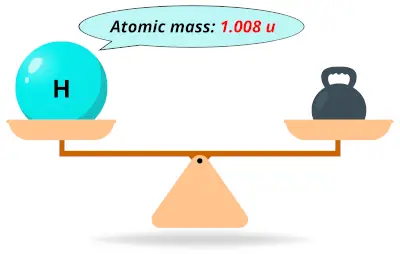 1.008 1.008 | 1 |
| 2 | Atomic mass of Helium (He) | 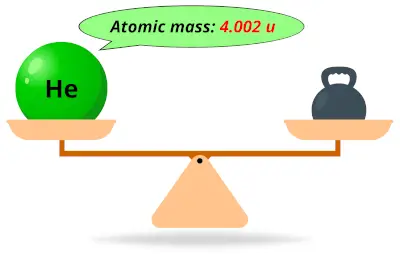 4.002 4.002 | 4 |
| 3 | Atomic mass of Lithium (Li) | 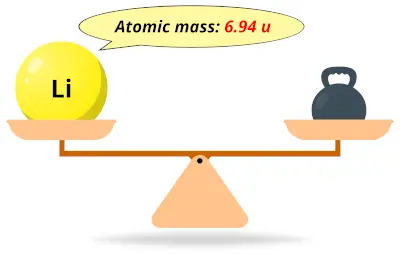 6.94 6.94 | 7 |
| 4 | Atomic mass of Beryllium (Be) | 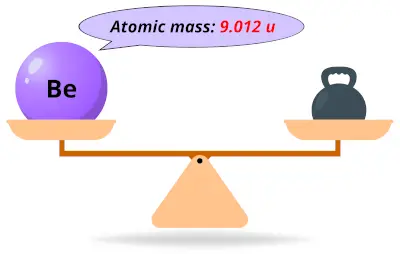 9.012 9.012 | 9 |
| 5 | Atomic mass of Boron (B) | 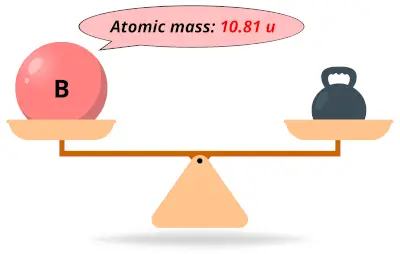 10.81 10.81 | 11 |
| 6 | Atomic mass of Carbon (C) | 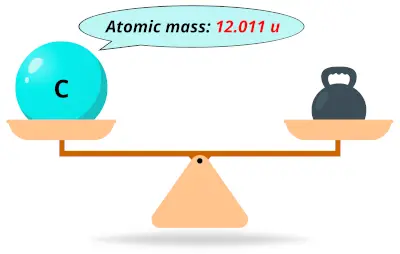 12.011 12.011 | 12 |
| 7 | Atomic mass of Nitrogen (N) | 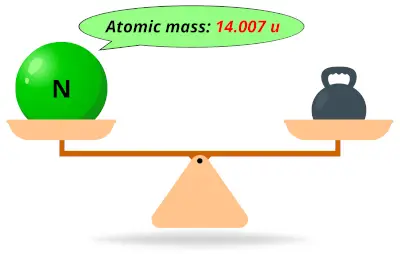 14.007 14.007 | 14 |
| 8 | Atomic mass of Oxygen (O) | 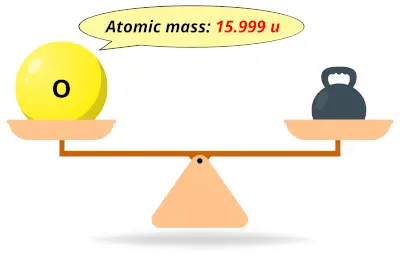 15.999 15.999 | 16 |
| 9 | Atomic mass of Fluorine (F) | 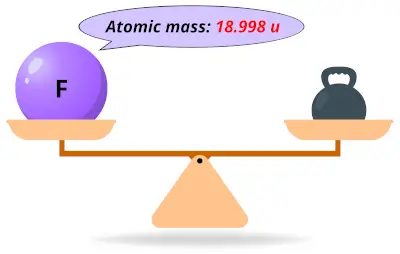 18.998 18.998 | 19 |
| 10 | Atomic mass of Neon (Ne) | 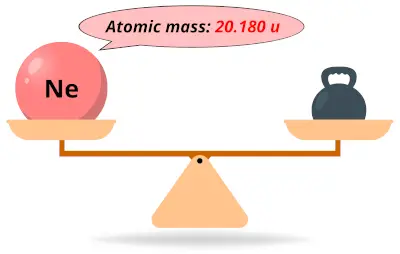 20.180 20.180 | 20 |
| 11 | Atomic mass of Sodium (Na) | 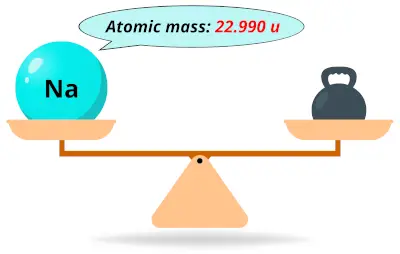 22.990 22.990 | 23 |
| 12 | Atomic mass of Magnesium (Mg) | 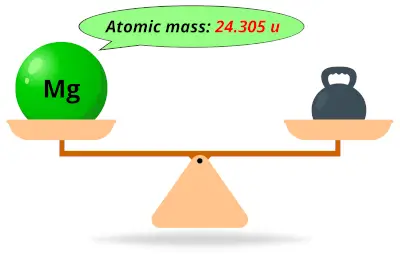 24.305 24.305 | 24 |
| 13 | Atomic mass of Aluminum (Al) | 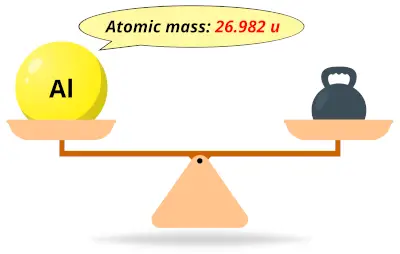 26.982 26.982 | 27 |
| 14 | Atomic mass of Silicon (Si) | 28 | |
| 15 | Atomic mass of Phosphorus (P) | 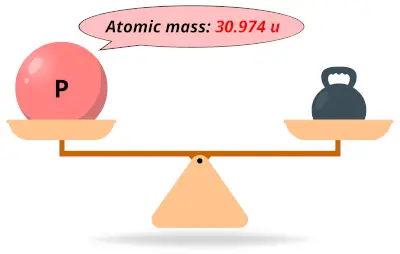 30.974 30.974 | 31 |
| 16 | Atomic mass of Sulfur (S) | 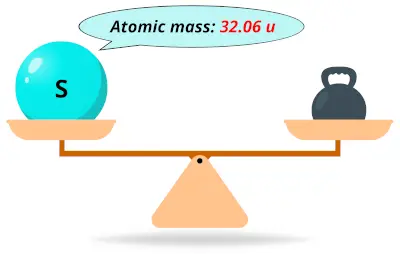 32.06 32.06 | 32 |
| 17 | Atomic mass of Chlorine (Cl) | 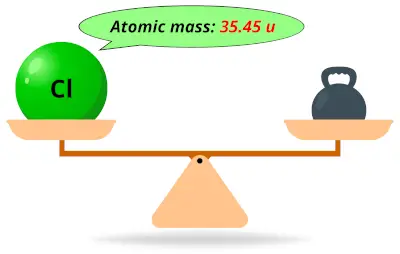 35.45 35.45 | 35 |
| 18 | Atomic mass of Argon (Ar) | 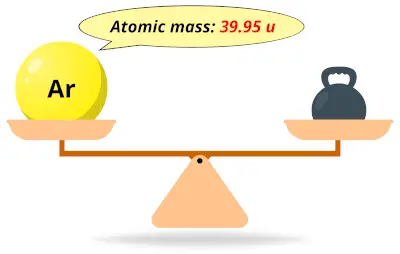 39.95 39.95 | 40 |
| 19 | Atomic mass of Potassium (K) | 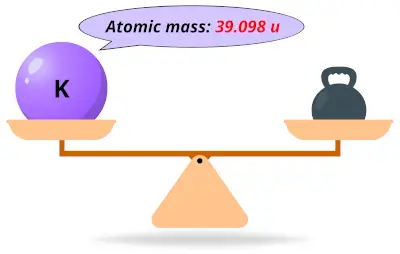 39.098 39.098 | 39 |
| 20 | Atomic mass of Calcium (Ca) | 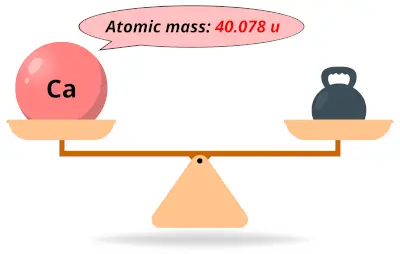 40.078 40.078 | 40 |
| 21 | Atomic mass of Scandium (Sc) | 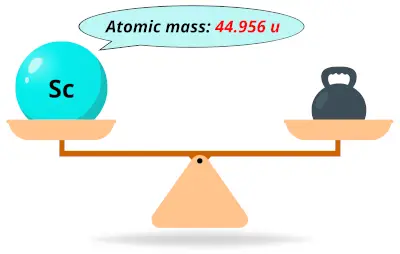 44.956 44.956 | 45 |
| 22 | Atomic mass of Titanium (Ti) | 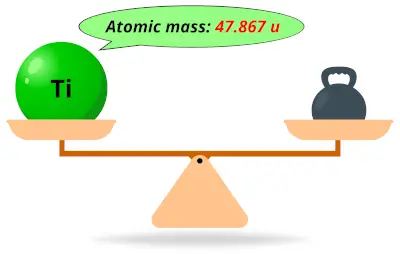 47.867 47.867 | 48 |
| 23 | Atomic mass of Vanadium (V) | 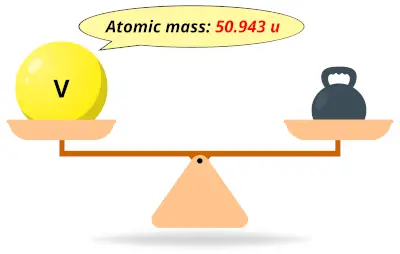 50.942 50.942 | 51 |
| 24 | Atomic mass of Chromium (Cr) | 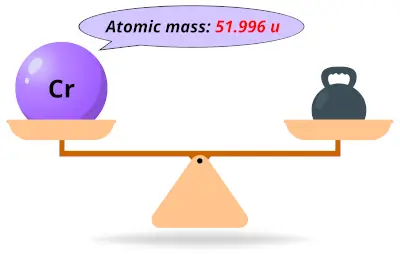 51.996 51.996 | 52 |
| 25 | Atomic mass of Manganese (Mn) | 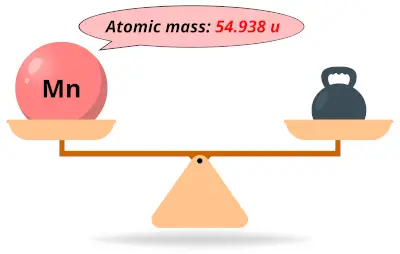 54.938 54.938 | 55 |
| 26 | Atomic mass of Iron (Fe) | 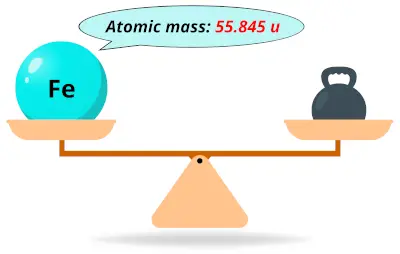 55.845 55.845 | 56 |
| 27 | Atomic mass of Cobalt (Co) | 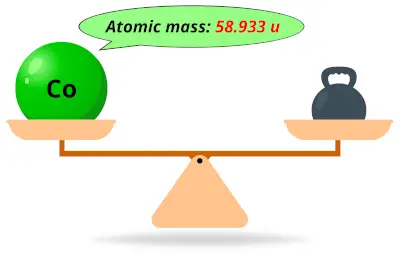 58.933 58.933 | 59 |
| 28 | Atomic mass of Nickel (Ni) | 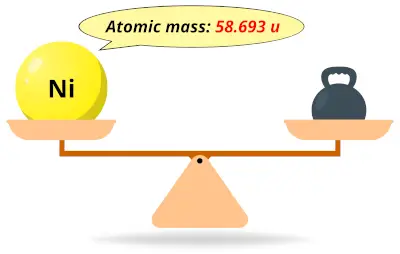 58.693 58.693 | 59 |
| 29 | Atomic mass of Copper (Cu) | 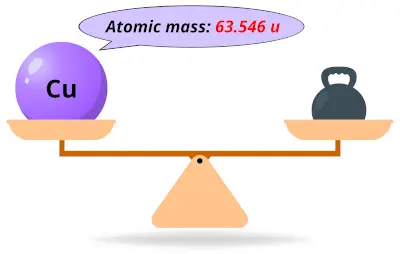 63.546 63.546 | 64 |
| 30 | Atomic mass of Zinc (Zn) | 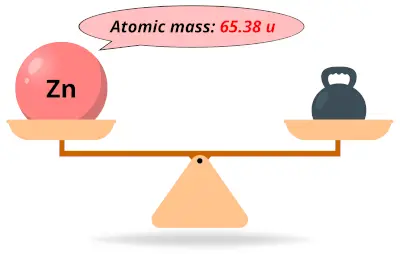 65.38 65.38 | 65 |
| 31 | Atomic mass of Gallium (Ga) | 69.723 | 70 |
| 32 | Atomic mass of Germanium (Ge) | 72.630 | 73 |
| 33 | Atomic mass of Arsenic (As) | 74.922 | 75 |
| 34 | Atomic mass of Selenium (Se) | 78.971 | 79 |
| 35 | Atomic mass of Bromine (Br) | 79.904 | 80 |
| 36 | Atomic mass of Krypton (Kr) | 83.798 | 84 |
| 37 | Atomic mass of Rubidium (Rb) | 85.468 | 85 |
| 38 | Atomic mass of Strontium (Sr) | 87.62 | 88 |
| 39 | Atomic mass of Yttrium (Y) | 88.906 | 89 |
| 40 | Atomic mass of Zirconium (Zr) | 91.224 | 91 |
| 41 | Atomic mass of Niobium (Nb) | 92.906 | 93 |
| 42 | Atomic mass of Molybdenum (Mo) | 95.95 | 96 |
| 43 | Atomic mass of Technetium (Tc) | (98) | (98) |
| 44 | Atomic mass of Ruthenium (Ru) | 101.07 | 101 |
| 45 | Atomic mass of Rhodium (Rh) | 102.91 | 103 |
| 46 | Atomic mass of Palladium (Pd) | 106.42 | 106 |
| 47 | Atomic mass of Silver (Ag) | 107.87 | 108 |
| 48 | Atomic mass of Cadmium (Cd) | 112.41 | 112 |
| 49 | Atomic mass of Indium (In) | 114.82 | 115 |
| 50 | Atomic mass of Tin (Sn) | 118.71 | 119 |
| 51 | Atomic mass of Antimony (Sb) | 121.76 | 122 |
| 52 | Atomic mass of Tellurium (Te) | 127.60 | 128 |
| 53 | Atomic mass of Iodine (I) | 126.90 | 127 |
| 54 | Atomic mass of Xenon (Xe) | 131.29 | 131 |
| 55 | Atomic mass of Cesium (Cs) | 132.91 | 133 |
| 56 | Atomic mass of Barium (Ba) | 137.33 | 137 |
| 57 | Atomic mass of Lanthanum (La) | 138.91 | 139 |
| 58 | Atomic mass of Cerium (Ce) | 140.12 | 140 |
| 59 | Atomic mass of Praseodymium (Pr) | 140.91 | 141 |
| 60 | Atomic mass of Neodymium (Nd) | 144.24 | 144 |
| 61 | Atomic mass of Promethium (Pm) | 145.00 | 145 |
| 62 | Atomic mass of Samarium (Sm) | 150.36 | 150 |
| 63 | Atomic mass of Europium (Eu) | 151.96 | 152 |
| 64 | Atomic mass of Gadolinium (Gd) | 157.25 | 157 |
| 65 | Atomic mass of Terbium (Tb) | 158.93 | 159 |
| 66 | Atomic mass of Dysprosium (Dy) | 162.50 | 163 |
| 67 | Atomic mass of Holmium (Ho) | 164.93 | 165 |
| 68 | Atomic mass of Erbium (Er) | 167.26 | 167 |
| 69 | Atomic mass of Thulium (Tm) | 168.93 | 169 |
| 70 | Atomic mass of Ytterbium (Yb) | 173.05 | 173 |
| 71 | Atomic mass of Lutetium (Lu) | 174.97 | 175 |
| 72 | Atomic mass of Hafnium (Hf) | 178.49 | 178 |
| 73 | Atomic mass of Tantalum (Ta) | 180.95 | 181 |
| 74 | Atomic mass of Tungsten (W) | 183.84 | 184 |
| 75 | Atomic mass of Rhenium (Re) | 186.20 | 186 |
| 76 | Atomic mass of Osmium (Os) | 190.23 | 190 |
| 77 | Atomic mass of Iridium (Ir) | 192.22 | 192 |
| 78 | Atomic mass of Platinum (Pt) | 195.08 | 195 |
| 79 | Atomic mass of Gold (Au) | 196.97 | 197 |
| 80 | Atomic mass of Mercury (Hg) | 200.59 | 201 |
| 81 | Atomic mass of Thallium (Tl) | 204.38 | 204 |
| 82 | Atomic mass of Lead (Pb) | 207.2 | 207 |
| 83 | Atomic mass of Bismuth (Bi) | 208.98 | 209 |
| 84 | Atomic mass of Polonium (Po) | (209) | (209) |
| 85 | Atomic mass of Astatine (At) | (210) | (210) |
| 86 | Atomic mass of Radon (Rn) | (222) | (222) |
| 87 | Atomic mass of Francium (Fr) | (223) | (223) |
| 88 | Atomic mass of Radium (Ra) | (226) | (226) |
| 89 | Atomic mass of Actinium (Ac) | (227) | (227) |
| 90 | Atomic mass of Thorium (Th) | 232.04 | 232 |
| 91 | Atomic mass of Protactinium (Pa) | 231.04 | 231 |
| 92 | Atomic mass of Uranium (U) | 238.03 | 238 |
| 93 | Atomic mass of Neptunium (Np) | (237) | (237) |
| 94 | Atomic mass of Plutonium (Pu) | (244) | (244) |
| 95 | Atomic mass of Americium (Am) | (243) | (243) |
| 96 | Atomic mass of Curium (Cm) | (247) | (247) |
| 97 | Atomic mass of Berkelium (Bk) | (247) | (247) |
| 98 | Atomic mass of Californium (Cf) | (251) | (251) |
| 99 | Atomic mass of Einsteinium (Es) | (252) | (252) |
| 100 | Atomic mass of Fermium (Fm) | (257) | (257) |
| 101 | Atomic mass of Mendelevium (Md) | (258) | (258) |
| 102 | Atomic mass of Nobelium (No) | (259) | (259) |
| 103 | Atomic mass of Lawrencium (Lr) | (266) | (266) |
| 104 | Atomic mass of Rutherfordium (Rf) | (267) | (267) |
| 105 | Atomic mass of Dubnium (Db) | (268) | (268) |
| 106 | Atomic mass of Seaborgium (Sg) | (269) | (269) |
| 107 | Atomic mass of Bohrium (Bh) | (270) | (270) |
| 108 | Atomic mass of Hassium (Hs) | (277) | (277) |
| 109 | Atomic mass of Meitnerium (Mt) | (278) | (278) |
| 110 | Atomic mass of Darmstadtium (Ds) | (281) | (281) |
| 111 | Atomic mass of Roentgenium (Rg) | (282) | (282) |
| 112 | Atomic mass of Copernicium (Cn) | (285) | (285) |
| 113 | Atomic mass of Nihonium (Nh) | (286) | (286) |
| 114 | Atomic mass of Flerovium (Fl) | (289) | (289) |
| 115 | Atomic mass of Moscovium (Mc) | (290) | (290) |
| 116 | Atomic mass of Livermorium (Lv) | (293) | (293) |
| 117 | Atomic mass of Tennessine (Ts) | (294) | (294) |
| 118 | Atomic mass of Oganesson (Og) | (294) | (294) |
Note:
- The Atomic masses are represented in the Atomic mass unit (u).
- The elements whose atomic masses are written in bracket ( ) are the synthetic elements and their atomic masses values represent the Atomic Mass of the most stable isotope.
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
