This is a SUPER easy guide on Titanium element.
In fact, the table mentioned below is the perfect information box (Which gives you every single detail about the Titanium element in Periodic table.)
So if you want to know anything about Titanium element, then this guide is for you.
Let’s finish this very quickly.
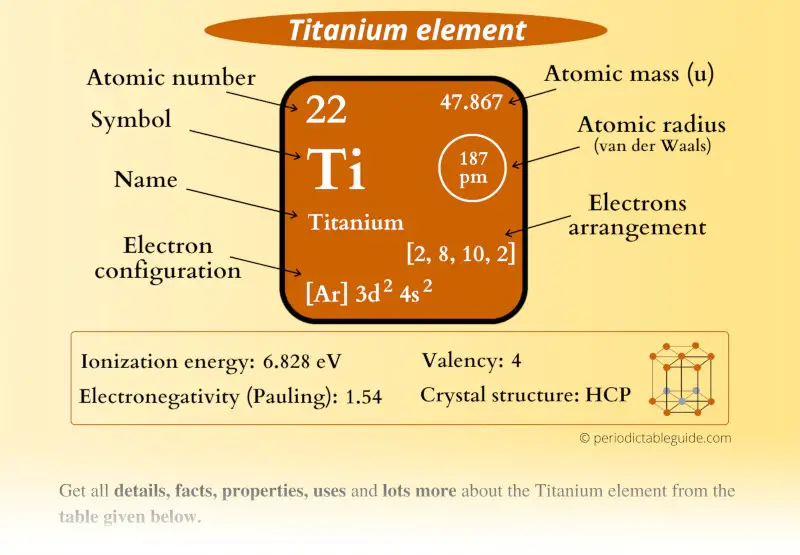
Titanium Element (Ti) Information
| Appearance |  Silvery white metallic color |
| State (at STP) | Solid |
| Position in Periodic table | 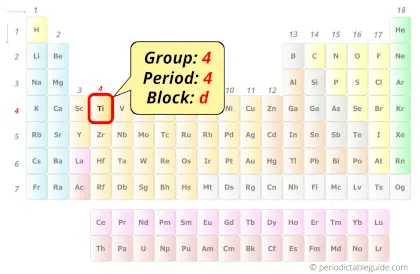 Group: 4, Period: 4, Block: d |
| Category | 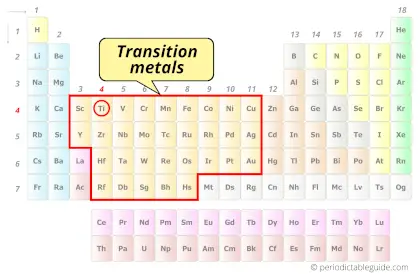 Transition metals |
| Atomic number or Protons | 22 |
| Neutrons | 26 |
| Electrons | 22 |
| Symbol | Ti |
| Atomic mass |  47.867 u |
| Electrons arrangement or Bohr model | 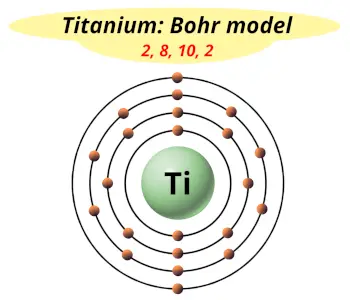 2, 8, 10, 2 |
| Electronic configuration | [Ar] 3d2 4s2 |
| Atomic radius | 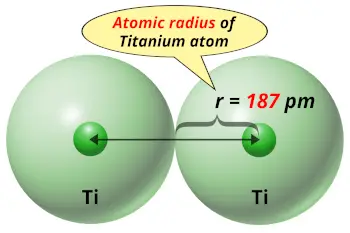 187 picometers (van der Waals radius) |
| 1st Ionization energy | 6.828 eV |
| Electronegativity | 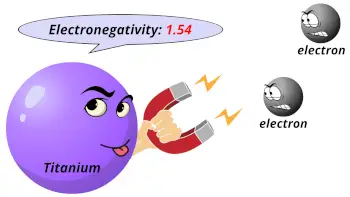 1.54 (Pauling scale) |
| Crystal structure | 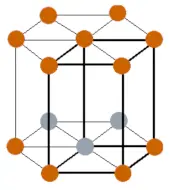 HCP (Hexagonal close packing) |
| Melting point | 1941 K or 1668 °C or 3034 °F |
| Boiling point | 3560 K or 3287 °C or 5949 °F |
| Density | 4.507 g/cm3 |
| Main isotope | 48Ti |
| Who discovered Titanium and when? | William Gregor in 1791 |
| CAS number | 7440-32-6 |
Titanium in Periodic table
Titanium element is in group 4 and period 4 of the Periodic table. Titanium is the d-block element and it belongs to transition metals group.
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba | La* | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| *Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| **Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
←Move to: Scandium (Sc) element – Periodic Table
→Move to: Vanadium (V) element – Periodic Table
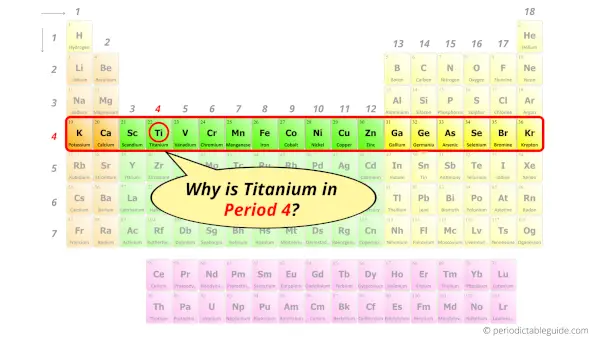
Why is Titanium in Period 4?
Let me ask you a question.
How many shells does titanium have?
It’s 4. Right?
You have already seen the bohr model of titanium atom in the above table.
From the Bohr model, it can be found that the number of orbits or shells in titanium is 4. Hence, as titanium has 4 orbits, it lies in period 4 of the Periodic table.
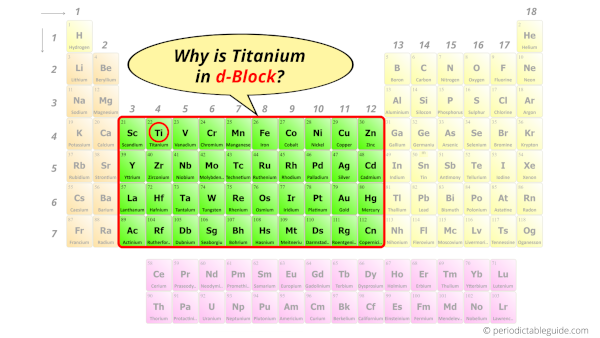
Why is Titanium in d-block?
Before knowing this reason, first of all I want to ask you a simple question.
How can you determine the blocks-wise position of elements?
The simple answer: The elements will lie in the s, p, d or f block will completely depend upon the subshell in which the last electron will enter.
For example; the electron configuration of titanium is [Ar] 4s2 3d2.
So the last electron of titanium enters the d-subshell or d-orbital.
Hence, titanium is the d-block element.
Is Titanium a Transition Metal? Why?
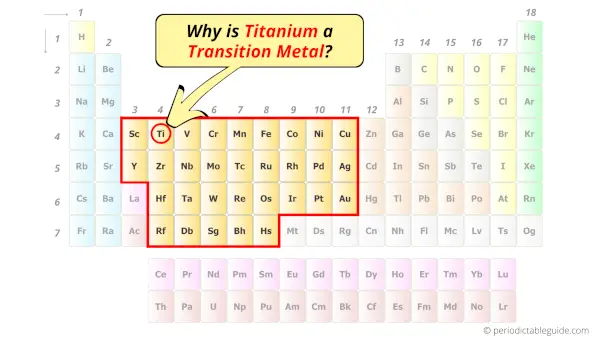
Yes, Titanium is a transition metal because it has incompletely filled d-orbital in its ground state.
Let me explain the exact meaning of this.
According to the definition of transition metals;
The element should compulsorily have incomplete d-orbitals, either in their ground state (M) or most common oxidation states (M1+, M2+, etc) then only they are called transition metals.
Now, the ground state of Titanium means its normal state in which it has neither gained nor lost any electron/s.
So the ground state of Titanium is Ti.
And the ground state electronic configuration of Titanium is [Ar] 4s2 3d2.
In this state, if we see the electron configuration of Titanium, then it possesses incomplete d-orbitals.
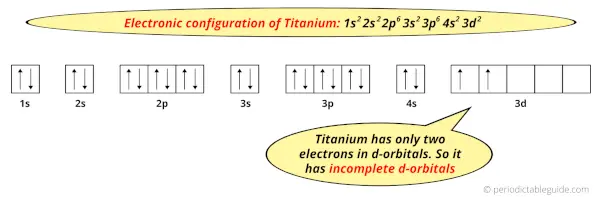
Because, there are only two electrons in the d-orbitals.
In order to have the complete d-orbitals, there must be 10 electrons in it.
But in the ground state electronic configuration of titanium, you can see that it has only 2 electrons in d-orbitals.
Thus, Titanium has incomplete d-orbitals.
And hence, as Titanium has incomplete d-orbitals, it is considered as a transition metal.
7 Interesting facts about Titanium
Interesting facts about titanium element are mentioned below.
- The name titanium was derived from the greek word “Titan” (from greek mythology).
- Titanium is the 9th most abundant element found in the earth’s crust.
- Titanium is mostly found in the igneous rocks on the earth.
- Strength of titanium is approximately twice the strength of aluminium.
- Titanium is 45% lighter than steel but has similar strength like steel.
- Titanium element is also present in the human body.
- Russia and China are the leading producers of titanium in the world.
Properties of Titanium
The physical and chemical properties of titanium element are mentioned below.
Physical properties of Titanium
Physical properties of titanium are mentioned below.
- Titanium is a metal having a silvery white metallic color.
- Density of titanium is 4.507 g/cm3 which is 60% denser than aluminum.
- The melting point of titanium is 1668 °C and its boiling point is 3287 °C.
- The crystal structure of titanium is HCP (Hexagonal close packing).
- Titanium has many isotopes, but out of those isotopes, the 48Ti is the most abundant (around 74%).
Chemical properties of Titanium
Chemical properties of titanium are mentioned below.
- Titanium is reactive metal and so it is not found in free state but it exists as a compound with other elements.
- Titanium is resistive to corrosion caused by saline water.
- Titanium is a transition metal having incomplete d-orbitals.
Uses of Titanium
Uses of titanium are mentioned below.
- Titanium has many uses, but most of the purified titanium is used in making a compound titanium dioxide (TiO2).
- Titanium dioxide (a compound of titanium) is used in making of paint, sunscreen, paper, cosmetics, etc as a white pigment.
- As titanium is anti corrosive, the titanium containers are used to store nuclear waste.
- Titanium is also added to pure gold which gives strength to the pure 24 karat gold.
- The medical industries as well as automobile and aerospace industries also use titanium.
- Besides these, titanium is also used to make bars, pipes, rods, tubes, wires, plates , etc.
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
External resources:
- P. (n.d.). Titanium | Ti (Element) – PubChem. Titanium | Ti (Element) – PubChem. https://pubchem.ncbi.nlm.nih.gov/element/Titanium
- It’s Elemental – The Element Titanium. (n.d.). It’s Elemental – the Element Titanium. https://education.jlab.org/itselemental/ele022.html
- Titanium – Wikipedia. (2022, January 19). Titanium – Wikipedia. https://en.wikipedia.org/wiki/Titanium
- Titanium – Element information, properties and uses | Periodic Table. (n.d.). Titanium – Element Information, Properties and Uses | Periodic Table. https://www.rsc.org/periodic-table/element/22/titanium
- Titanium. (n.d.). Titanium. https://webbook.nist.gov/cgi/inchi?ID=C7440326&Mask=20
